DIP vs. STR Markers in Forensic Science: A Comprehensive Analysis for Advanced DNA Profiling
This article provides a comparative analysis of Deletion/Insertion Polymorphism (DIP) panels and Short Tandem Repeat (STR) markers for forensic genetic applications.
DIP vs. STR Markers in Forensic Science: A Comprehensive Analysis for Advanced DNA Profiling
Abstract
This article provides a comparative analysis of Deletion/Insertion Polymorphism (DIP) panels and Short Tandem Repeat (STR) markers for forensic genetic applications. Tailored for researchers and forensic development professionals, it explores the foundational principles of both marker types, detailing their methodological applications in personal identification, ancestry inference, and resolving challenging DNA mixtures. The scope includes troubleshooting common analytical challenges, optimizing protocols for degraded samples, and a critical validation of performance metrics. By synthesizing current research and validation studies, this analysis serves as a strategic guide for selecting appropriate genetic markers to advance the capabilities of forensic and biomedical genotyping.
Core Principles: Understanding DIP and STR Genetic Architectures
Short Tandem Repeats (STRs) are repetitive DNA sequences, typically 2 to 7 base pairs in length, that are highly variable among individuals. For decades, the analysis of a core set of STRs using Polymerase Chain Reaction (PCR) followed by Capillary Electrophoresis (CE) has been the definitive method for human identification in forensic genetics, paternity testing, and disaster victim identification. [1] Their exclusivity and high discrimination power have made them indispensable. However, the analysis of unbalanced DNA mixtures—where one contributor's DNA is vastly exceeded by another's—represents a significant limitation of conventional STR profiling. [1] [2]
This guide provides a comparative analysis of traditional STR markers against emerging Deletion/Insertion Polymorphism (DIP) panels, focusing on their performance in resolving forensic mixtures.
Experimental Protocols and Performance Data
Standard STR Analysis Protocol
The standard protocol for forensic STR analysis involves the multiplex amplification of typically 20-21 autosomal STR loci, plus the amelogenin sex marker, from DNA extracts using commercial kits, followed by fragment size separation and detection via capillary electrophoresis. [3]
Core Limitation: In a mixture from two individuals, the minor contributor's profile cannot be successfully detected if its DNA share is less than approximately 5-10% of the total. The major contributor's profile "masks" that of the minor contributor, making interpretation difficult or impossible. [1] [2]
DIP-STR Marker Analysis
DIP-STRs are compound markers designed specifically to target the minor component in an unbalanced mixture. [1] [4] The method uses allele-specific PCR primers targeting a DIP (Deletion/Insertion Polymorphism) region to selectively amplify one haplotype, which is then sub-typed by a linked STR. This design suppresses the amplification of the dominant DNA.
Key Experimental Steps: [1] [4]
- Marker Selection: Bioinformatic selection of DIP-STR markers where the DIP and STR are in close proximity.
- PCR Amplification: Two separate PCR reactions are often performed using primers specific to the insertion ("L" allele) or deletion ("S" allele) of the DIP.
- Capillary Electrophoresis: The amplified fragments are separated and detected, similar to conventional STRs, with the STR alleles providing the discrimination power.
Experimental Workflow: STRs vs. DIP-STRs
The diagram below illustrates the key procedural differences between standard STR and DIP-STR analyses for mixture deconvolution.
Quantitative Performance Comparison
The table below summarizes key performance characteristics of standard STRs, DIP-STRs, and other relevant markers based on experimental data.
| Feature | Standard Autosomal STRs | DIP-STR Markers | Y-STR Markers |
|---|---|---|---|
| Primary Forensic Use | Individual identification, paternity testing [1] | Deconvoluting extremely unbalanced two-person mixtures [1] [4] | Identifying male DNA in female-rich mixtures; patrilineal lineage [1] [5] |
| Typical Detection Limit for Minor DNA | 1:10 to 1:20 (5-10% of total) [2] | Up to 1:1000 (0.1% of total) [1] [4] | Varies, but effective in sexual assault evidence [1] |
| Key Advantage | High discrimination power, standardized kits, extensive databases | Extreme sensitivity for minor contributor, gender-independent [1] | Effective for male-specific profiling in mixtures [5] |
| Key Limitation | Fails in highly unbalanced mixtures due to masking [1] | Requires pre-defined marker panels; informative genotypes depend on allele mismatch [1] [2] | Limited to male contributors; cannot distinguish closely related males [1] |
| Mutation Rate | Relatively high (order of 10-3) [1] | Lower than STRs [1] | Standard Y-STRs: Low; Rapidly Mutating (RM) Y-STRs: High [5] |
| Method Compatibility | PCR + Capillary Electrophoresis [1] | PCR + Capillary Electrophoresis [1] | PCR + Capillary Electrophoresis [5] |
The Scientist's Toolkit: Essential Research Reagents
The table below lists key reagents and materials required for conducting STR and DIP-STR analyses in a research or forensic validation context.
| Reagent / Material | Function in Experiment |
|---|---|
| Commercial STR Kits (e.g., Investigator 24plex QS, PowerPlex Fusion 6C) [3] | Provides optimized primer mixes, master mix, and controls for multiplex amplification of core STR loci. |
| DIP-STR Primer Panels [4] [6] | Custom-designed allele-specific primers for targeted amplification of DIP-STR loci. |
| Thermostable DNA Polymerase | Enzyme for PCR amplification of target STR/DIP-STR regions. |
| Capillary Electrophoresis Instrument (e.g., 3500 Genetic Analyzer) [3] | For separation, detection, and sizing of fluorescently labeled PCR fragments. |
| Genetic Analyzer Software (e.g., Gene Mapper ID-X) [3] | Software for analyzing CE data, assigning allele calls, and generating genetic profiles. |
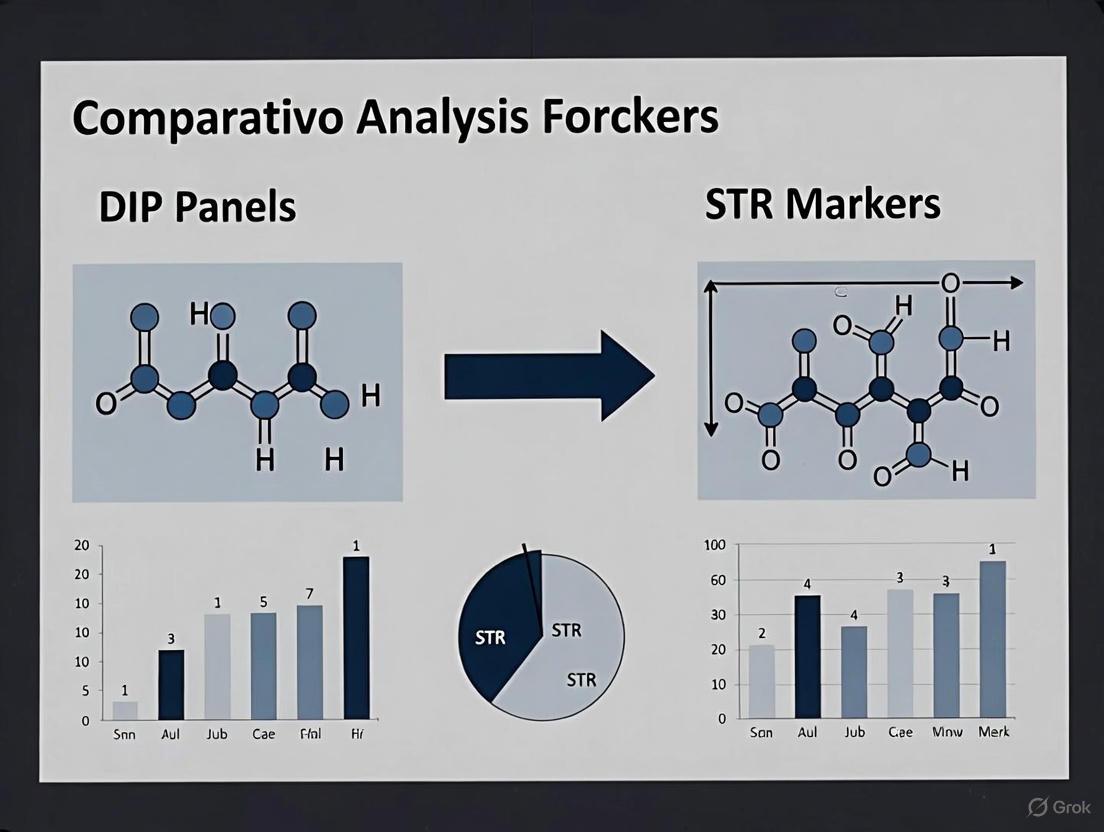
Structural Insights: Marker Architecture
The fundamental difference in marker design between a standard STR and a compound DIP-STR is key to their performance.
Future Directions
While DIP panels and DIP-STR markers show superior performance for specific challenging samples like extremely unbalanced mixtures, traditional STRs remain the workhorse for the vast majority of forensic casework due to their well-established protocols, population databases, and high power of discrimination for single-source and moderately mixed samples. [2] [3] The future of forensic genetics lies in selecting the right tool from an expanding toolkit—which includes STRs, DIPs, SNPs, and sequencing technologies—to meet the specific demands of each evidentiary sample.
In the field of forensic genetics, the comparative analysis of DNA marker technologies is fundamental to advancing investigative capabilities. This guide provides a structured comparison between Deletion/Insertion Polymorphisms (DIPs) and the established standard of Short Tandem Repeats (STRs), focusing on their structure, stability, and performance in forensic applications. DIPs, also known as insertion/deletion (InDel) polymorphisms, are bi-allelic markers representing the presence or absence of specific DNA sequences, typically ranging from 1 to 20 base pairs (bp) in length [7]. Their structural simplicity and low mutation rate (estimated on the order of 10⁻⁸) confer significant analytical advantages, including minimal stutter peaks and robustness in analyzing degraded DNA [7]. As the forensic community continues to seek more reliable and informative genotyping methods, understanding the comparative performance of DIP panels versus STR markers becomes essential for researchers, scientists, and drug development professionals evaluating the next generation of forensic DNA analysis tools.
Structural Characteristics and Marker Comparison
The fundamental differences between DIPs and STRs originate from their distinct molecular structures and mutational mechanisms. STRs, or microsatellites, consist of tandemly repeating motifs of 2-6 bp, with polymorphisms arising from variations in the number of repeat units due to strand-slippage during DNA replication [8]. This repetitive structure makes them highly polymorphic but also prone to PCR artifacts like stutter peaks, which can complicate mixture interpretation. In contrast, DIPs are bi-allelic markers (either insertion or deletion alleles) at a specific genomic location, resulting in more stable amplification profiles with no stutter peaks when examined by PCR-CE platforms [7].
Table 1: Structural and Technical Comparison of DIPs and STRs
| Characteristic | DIP Markers | STR Markers |
|---|---|---|
| Molecular Structure | Presence/absence of specific sequences (1-20 bp) | Tandem repeats of 2-6 bp motifs |
| Allelic Nature | Primarily bi-allelic | Multi-allelic |
| Mutation Rate | Low (~10⁻⁸) [7] | Higher (10⁻³ to 10⁻⁵) |
| PCR Artifacts | No stutter peaks [7] | Significant stutter peaks |
| Typing Method | PCR-CE or NGS platforms [7] | Primarily PCR-CE |
| Amplicon Size | Can be optimized <200 bp for degraded DNA [7] | Typically longer (100-500 bp) |
Compound markers like DIP-STRs have been developed to leverage the advantages of both systems. These markers pair a DIP with a nearby STR, creating a highly polymorphic haplotype that enables the analysis of extremely unbalanced DNA mixtures (up to 1:1000 minor:major ratio) [4] [9]. The DIP component allows for allele-specific priming to target the minor contributor, while the STR provides high discrimination power, overcoming limitations of standard STR profiling in mixture analysis [4].
Performance Comparison: Experimental Data
Validation studies directly comparing DIP panels and STR markers provide critical performance metrics for objective assessment. A recently developed 60-DIP panel demonstrated robust performance in forensic testing, with a combined probability of discrimination of 0.999999999999 and cumulative probability of paternity exclusion of 0.9937 for East Asian populations [7] [10]. The panel's performance with degraded samples was particularly notable, as amplicons were limited to 200 bp to accommodate compromised DNA quality [7].
Table 2: Quantitative Performance Metrics of DIP Panels vs. STRs
| Performance Metric | 60-DIP Panel Performance | Typical STR Panel Performance | Experimental Context |
|---|---|---|---|
| Combined PD | 0.999999999999 [7] | >0.999999999 (for 20+ loci) | Personal identification power |
| Combined PE | 0.9937 [7] | >0.9999 (for 20+ loci) | Paternity testing |
| Mixture Resolution | DIP-STRs effective at 1:1000 ratios [4] [9] | Limited beyond 1:10-1:20 ratios | Unbalanced two-source mixtures |
| Degraded DNA Analysis | Excellent (amplicons <200 bp) [7] | Variable (amplicons 100-500 bp) | Forensic casework samples |
| Ancestry Inference | Effective for East Asian subgroups [7] | Limited without additional SNPs | Biogeographic analysis |
For mixture deconvolution, DIP-STR markers show remarkable sensitivity, successfully genotyping minor contributors representing only 0.1% of mixed stains [9]. This performance significantly surpasses standard STR analysis, which typically requires the minor contributor to represent at least 10-20% of the total DNA for reliable detection [4]. The compound nature of DIP-STR markers generates high-level polymorphism suitable for identity testing while maintaining the stability of DIP markers, making them particularly valuable for challenging forensic samples including touch DNA [9].
Experimental Protocols and Methodologies
DIP Panel Validation Protocol
The developmental validation of DIP panels follows guidelines recommended by the Scientific Working Group on DNA Analysis Methods (SWGDAM) [7]. The standard protocol involves:
Marker Selection: DIPs are selected from databases like the 1000 Genomes Project with minimum allele frequency (MAF) ≥0.1, ensuring they are bi-allelic with length variations of 1-20 bp and located on different chromosomes or >5 Mb apart on the same chromosomal arm [7].
PCR Optimization: Reaction conditions are systematically optimized including primer mix concentration (0.5× to 1.5×), reaction volume (5-25 μL), denaturation temperature (89-99°C), annealing temperature (55-65°C), and cycle number (21-27 cycles) [7].
Capillary Electrophoresis: Amplified products are separated and detected using multi-capillary electrophoresis systems with a 6-dye chemistry [7].
Validation Parameters: Comprehensive tests include sensitivity (down to 0.06 ng DNA), species specificity, stability, mixture analysis, reproducibility, and case sample studies [7] [9].
DIP-STR Mixture Deconvolution Protocol
For resolving extremely unbalanced mixtures using DIP-STR markers:
Primer Design: Two allele-specific primers are designed - one for the deletion (S-DIP primer) and one for the insertion (L-DIP primer) - paired with a non-allele specific STR primer [4] [9].
Allele-Specific Amplification: Under informative genotypes, allele-specific primers selectively amplify the minor DNA contributor in a mixture, overcoming the masking effect of the major DNA [4].
Haplotype Analysis: The paired STR primer enables full STR region coverage, generating a polymorphic haplotype for high discrimination power [9].
Analysis: Capillary electrophoresis or massively parallel sequencing detects the haplotypes, with sensitivity validated for minor contributors at 0.1% of the mixture [9].
Figure 1: Comparative Workflows for DIP, DIP-STR, and STR Analysis
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions for DIP and STR Analysis
| Reagent/Resource | Function | Example Application |
|---|---|---|
| 1000 Genomes Project Database | Source of population-specific DIP frequencies | Marker selection and ancestry inference [7] |
| Primer Premier 5.0 Software | Primer design and evaluation | Developing multiplex DIP panels [7] |
| AutoDimer Software | Primer dimer evaluation | Optimizing multiplex PCR conditions [7] |
| STRAF Software | Population genetic parameters | Calculating forensic statistical parameters [7] |
| GENEPOP 4.0 | HWE and linkage disequilibrium testing | Population genetics statistics [7] |
| STRUCTURE v2.3.4 | Genetic structure analysis | Ancestry component determination [7] |
| gnomAD Database | Genome aggregation of variants | Identifying novel DIP-STR candidates [9] |
| PowerSeq 46GY System | Commercial STR multiplex kit | Comparative STR analysis [11] |
| Oxford Nanopore MinION | Portable sequencing platform | Sequence-based STR and DIP analysis [11] |
The comparative analysis of DIP panels versus STR forensic markers reveals distinct advantages for specific applications. DIP markers offer superior stability, lower mutation rates, and absence of stutter artifacts, making them particularly valuable for analyzing degraded DNA and biogeographic ancestry inference [7]. The recently validated 60-DIP panel demonstrates that DIPs can provide both high discrimination power for personal identification and valuable ancestry information for East Asian populations [10]. For mixture deconvolution, DIP-STR compound markers enable resolution of extremely unbalanced samples (up to 1:1000 ratios) where standard STR profiling fails [4] [9]. While STRs remain the gold standard for routine forensic DNA profiling due to established databases and high discrimination power with multi-allelic markers, DIP-based technologies represent a promising advancement for challenging forensic casework. The integration of DIP markers with emerging sequencing technologies like the Oxford Nanopore MinION platform further expands their potential for comprehensive forensic analysis [11]. As the field continues to evolve, DIP markers offer forensic researchers and scientists powerful complementary tools to address limitations of current STR-based systems, particularly for degraded samples, unbalanced mixtures, and ancestry inference.
The analysis of complex DNA mixtures represents one of the most significant challenges in modern forensic genetics. Conventional Short Tandem Repeat (STR) markers, while the gold standard for human identification, exhibit limited sensitivity in characterizing samples containing DNA from several contributors in very different proportions [4]. Typically, PCR fragment analysis allows detection of a minor DNA component only when it represents more than 10% of the total DNA, with unambiguous identification requiring a minor DNA fraction of at least 20% [4]. This limitation has profound implications for justice, particularly in cases where biological stains contain small quantities of a perpetrator's DNA mixed with a large amount of a victim's DNA [4].
To address this analytical gap, researchers have developed innovative compound genetic markers that combine different types of polymorphisms to enhance detection sensitivity and discriminatory power. Among the most promising are DIP-STR (Deletion/Insertion Polymorphism-Short Tandem Repeat) and SNP-STR (Single Nucleotide Polymorphism-Short Tandem Repeat) markers, which leverage the advantages of different genetic variations while mitigating their individual limitations [12]. These hybrid systems enable forensic scientists to resolve extremely unbalanced DNA mixtures that would otherwise be intractable with conventional methods, opening new possibilities for criminal investigations, paternity testing, and medical genetics.
Fundamental Principles of Compound Marker Systems
DIP-STR Markers: Architecture and Mechanism
The DIP-STR marker is a sophisticated analytical tool that pairs a deletion-insertion polymorphism (DIP) with a closely linked short tandem repeat (STR). This compound architecture generates a highly polymorphic haplotype suitable for identity testing [4]. The fundamental innovation lies in the primer design: two allele-specific PCR primers target either the deletion (short or "S" allele) or insertion (long or "L" allele) of the DIP, while a universal STR primer enables amplification of the adjacent repetitive region [1]. This strategic design allows for the unambiguous genotyping of a minor component in the presence of a major component at ratios up to 1:1,000 [4] [1].
The power of DIP-STR analysis derives from its ability to target genomic regions unique to the minor DNA source, thereby eliminating the masking effect of the major DNA [4]. The DIP component serves as an allele-specific anchor, while the linked STR provides additional discrimination power through its length polymorphisms. This dual-information system enables forensic geneticists to obtain complete genetic profiles from trace contributors in complex mixtures, a capability that has proven valuable in sexual assault cases where the perpetrator contributes only minimal DNA compared to the victim [9].
SNP-STR Markers: Structure and Analytical Approach
SNP-STR markers represent another compound system that combines a biallelic single nucleotide polymorphism (SNP) with a closely linked STR [1] [12]. Similar to DIP-STRs, these markers employ an allele-specific PCR approach based on the Amplification Refractory Mutation System (ARMS) principle, with SNP allele-specific primers labeled with different fluorescent dyes and positioned to amplify the adjacent STR region [1]. The forward and reverse primers are located upstream and downstream of the STR polymorphism sequence, respectively, ensuring co-amplification of the compound haplotype.
The analytical strength of SNP-STR markers lies in their ability to target distinctive alleles of the minor contributor in a DNA mixture, reducing the negative effect of masking by the dominant DNA [1]. By adding a deliberate mismatch at the 3' end of the primers, researchers can increase the specificity of PCR amplification in DNA mixtures, enhancing the signal from the minor contributor [1]. Although SNP-STRs generally offer lower sensitivity (typically 1:40 minor:major ratio) compared to DIP-STRs, they provide advantages in scenarios involving highly degraded DNA due to their shorter amplicon sizes [1] [12].
Table 1: Comparative Characteristics of Compound Marker Systems
| Feature | DIP-STR | SNP-STR | Conventional STR |
|---|---|---|---|
| Marker Composition | DIP + STR | SNP + STR | STR only |
| Sensitivity (Minor:Major Ratio) | Up to 1:1000 [1] | ~1:40 [1] | ~1:5-1:10 [4] |
| Polymorphism Type | Multiallelic (STR) + Biallelic (DIP) | Multiallelic (STR) + Biallelic (SNP) | Multiallelic (STR) |
| Amplicon Size | ~230 bp average [9] | Typically <550 bp [1] | Often >300 bp |
| Mutation Rate | Lower for DIP component | Lower for SNP component | Higher for STR component |
| Stutter Peaks | Reduced compared to STR alone | Reduced compared to STR alone | Common artifact |
| Population Data Availability | Limited but growing [9] [13] | Emerging [1] | Extensive |
Experimental Protocols and Methodological Considerations
DIP-STR Workflow and Analysis
The experimental workflow for DIP-STR analysis begins with DNA extraction from forensic samples using standard methods, followed by quantification and quality assessment [9]. The critical step involves allele-specific PCR amplification using primers designed to target either the insertion or deletion allele of the DIP component while simultaneously amplifying the flanking STR region. This targeted approach enables preferential amplification of the minor contributor's DNA when the major contributor lacks the targeted DIP allele [4].
After amplification, products are separated and detected using capillary electrophoresis (CE), similar to conventional STR analysis [1]. The resulting electrophoregrams are analyzed to determine DIP-STR haplotypes, with interpretation focusing on identifying informative patterns where the minor contributor possesses DIP alleles absent in the major contributor [1]. This methodology has been successfully applied to various challenging sample types, including "touch" DNA, degraded samples, and cell-free DNA from maternal plasma [9].
DIP-STR Analysis Workflow: This diagram illustrates the key steps in DIP-STR analysis, highlighting the allele-specific primer design that enables minor contributor detection.
SNP-STR Methodology
The SNP-STR protocol shares similarities with DIP-STR analysis but incorporates distinct primer design considerations for SNP targeting. The process begins with DNA extraction and quantification, followed by multiplex PCR amplification using SNP allele-specific primers labeled with different fluorescent dyes [1]. These primers are designed with deliberate mismatches at their 3' ends to enhance amplification specificity, particularly crucial when targeting minor DNA components in mixtures [1].
Following amplification, products undergo fragment separation by capillary electrophoresis and genotype calling based on both SNP and STR polymorphisms [1]. The analysis focuses on identifying informative combinations where the minor contributor possesses SNP alleles not present in the major contributor, enabling deconvolution of mixture components. Bioinformatic analyses and population databases play critical roles in selecting optimal SNP-STR markers with appropriate minor allele frequencies and linkage characteristics [1].
Performance Comparison and Experimental Data
Sensitivity in Unbalanced Mixture Detection
Compound markers demonstrate remarkable sensitivity in detecting minor DNA components in unbalanced mixtures, far exceeding the capabilities of conventional STR systems. Experimental data show that DIP-STR markers can successfully genotype a minor component at ratios up to 1:1,000 (0.1% minor DNA) when using informative allele combinations [4] [9]. This exceptional sensitivity enables forensic analysis in scenarios where the trace contributor would otherwise be undetectable, such as in touch DNA evidence or samples containing high background DNA from a primary contributor.
In comparison, SNP-STR markers typically achieve detection sensitivity of approximately 1:40 (2.5% minor DNA), which, while less sensitive than DIP-STRs, still represents a significant improvement over conventional STR systems [1]. This intermediate sensitivity makes SNP-STRs valuable in mixture scenarios with moderately unbalanced ratios, while DIP-STRs remain the preferred choice for extremely unbalanced samples. The difference in performance stems from the more pronounced sequence variation targeted by DIP primers compared to SNP primers, allowing for more specific amplification of the minor component [1].
Table 2: Experimental Performance Metrics of Compound Markers
| Performance Metric | DIP-STR | SNP-STR | Conventional STR |
|---|---|---|---|
| Detection Limit | 0.06 ng of DNA [9] | Not specified | Varies by kit |
| Minor Contributor Detection | 0.1% of mixture [9] | ~2.5% of mixture [1] | 5-10% of mixture [4] |
| Informativeness Rate | ~70% (varies by population) [1] | Based on MAF and STR variability [1] | N/A |
| Degraded DNA Performance | Excellent (short amplicons) [9] | Excellent (short amplicons) [12] | Moderate to poor |
| Mixture Deconvolution | Two-person mixtures [9] | Two-person mixtures [1] | Limited for unbalanced mixtures |
Population Variability and Forensic Parameters
The forensic utility of compound markers depends significantly on their polymorphism across different populations. Research on DIP-STR markers in Swiss populations revealed an average heterozygosity of 0.7 across 30 validated forensic markers, indicating high discrimination power [9]. Similarly, studies in US population groups including European-American, African-American, Hispanic, and Asian-American cohorts demonstrated differential distribution of DIP-STR alleles with distinct population-specific signatures [13]. This population structure information can provide valuable investigative leads in addition to identification capabilities.
For SNP-STR markers, the effective number of alleles and discrimination power depends on both the minor allele frequency (MAF) of the SNP component and the polymorphism of the STR component [1]. Optimal SNP-STR markers typically feature MAF values greater than 0.02 and amplicon lengths shorter than 550 bp to ensure adequate population coverage and performance with degraded DNA [1]. The compound nature of these markers creates haplotypes with significantly higher discrimination power than either component alone, enhancing their forensic utility.
Applications in Forensic and Medical Genetics
Forensic Casework Applications
The primary application of compound markers lies in forensic mixture deconvolution, particularly for sexual assault evidence where the perpetrator's DNA may be present in trace quantities compared to the victim's DNA [9]. In one demonstrated application, DIP-STR markers successfully characterized a minor DNA contributor when standard STRs failed to detect any contribution and Y-STR profiling could not differentiate between related male suspects [9]. This capability to generate complete genetic profiles from minimally represented contributors represents a significant advancement for justice systems worldwide.
Beyond routine mixture analysis, compound markers show promise for touch DNA evidence, where the limited quantity and quality of DNA often challenge conventional STR systems [9]. The shorter amplicon sizes of optimized DIP-STR markers (averaging 230 bp) enhance their performance with degraded samples, generating interpretable profiles where standard STR kits might fail [9]. Additionally, the absence of stutter peaks common in conventional STR analysis improves interpretation accuracy, reducing the risk of false allele calls [9].
Medical and Prenatal Applications
Compound markers have found important applications in medical genetics, particularly for noninvasive prenatal testing. DIP-STR markers can target fetal DNA circulating in maternal blood as early as eight weeks of pregnancy, enabling noninvasive paternity testing without risk to the fetus [1] [9]. In singleton pregnancies, the method can detect a single non-maternal DIP-STR allele inherited from the father, while in twin pregnancies (particularly dizygotic), it can identify two non-maternal DIP-STR alleles in the maternal plasma [1].
These markers also facilitate DNA microchimerism analysis in clinical contexts, such as detecting trace quantities of fetal DNA in maternal blood following pregnancy or donor DNA in transplant recipients [4]. The exceptional sensitivity of DIP-STR markers enables detection of these minor DNA populations at biologically relevant concentrations, supporting medical diagnostics and treatment monitoring in transplantation medicine [4].
Research Reagent Solutions and Technical Requirements
The implementation of compound marker analysis requires specific reagents and technical capabilities. Listed below are essential research solutions for laboratories developing these applications:
Allele-Specific Primers: Designed to target either insertion or deletion alleles (DIP-STR) or specific SNP alleles (SNP-STR) with deliberate 3' mismatches to enhance specificity [1].
Multiplex PCR Master Mix: Optimized for amplification of multiple compound markers simultaneously, often incorporating hot-start enzymes and enhanced buffer systems [9].
Capillary Electrophoresis System: Standard forensic genetic analyzers (e.g., ABI 3500) with appropriate fluorescent dye sets for multiplex detection [1] [14].
Population Databases: Curated genetic databases containing allele frequencies for compound markers across relevant populations for statistical interpretation [13].
Bioinformatic Tools: Specialized software for primer design, virtual binning, and data analysis to address unique challenges of compound markers [9].
Quality Control Materials: Reference DNA standards and control samples to validate assay performance and ensure genotyping accuracy [9].
Future Directions and Integration with Emerging Technologies
The field of compound marker analysis is rapidly evolving, with several promising directions emerging. Multiplex panel development represents an immediate priority, as current DIP-STR and SNP-STR analyses typically employ limited marker sets [9]. Research indicates that hundreds of DIP-STR candidates exist in the human genome, with recent studies validating additional markers to create more comprehensive panels [9]. Expanding these multiplex systems will enhance discrimination power and reduce the need for a priori selection of informative loci based on expected donor genotypes.
Integration with next-generation sequencing (NGS) platforms represents another significant frontier [12] [15]. While current compound marker analysis primarily uses capillary electrophoresis, transitioning to NGS would enable simultaneous analysis of multiple marker types (autosomal STRs, SNPs, DIPs) in a single reaction, providing deeper insights into complex mixtures [12]. The development of mini-haplotype (MiniHap) markers containing five or more SNPs demonstrates how NGS can facilitate even more sophisticated mixture deconvolution approaches [15].
Emerging technologies like CRISPR-based detection and nanopore sequencing further expand the potential applications of compound markers [12] [15]. These platforms may enable rapid, portable analysis of forensic samples in field settings, potentially revolutionizing crime scene investigation. However, significant validation work remains before these technologies can be adopted for routine forensic casework, particularly regarding standardization, reproducibility, and adherence to forensic quality assurance standards [12].
Population Genetics and Allele Frequency Diversity Across Global Cohorts
In the specialized field of forensic genetics, the analysis of biological mixtures containing DNA from multiple contributors presents a significant analytical challenge. For decades, Short Tandem Repeats (STRs) have been the gold standard for human identification, paternity testing, and population genetic studies due to their high polymorphism [1]. However, a fundamental limitation of STRs is their poor performance in characterizing unbalanced DNA mixtures where one contributor's DNA is present in trace amounts relative to another [4]. When the minor DNA component constitutes less than 10% of the total DNA, standard STR analysis often fails to detect it due to PCR amplification bias and the masking effect of the major contributor's profile [2]. This limitation has profound implications for forensic investigations involving mixed stains and medical genetics research addressing DNA microchimerism in pregnancy or post-transplant patients [4].
To address these challenges, novel genetic markers have emerged that combine different types of polymorphisms to enhance sensitivity and discrimination power. Among these, Deletion/Insertion Polymorphisms (DIPs) and compound markers like DIP-STRs represent promising alternatives that leverage the advantages of both DIP and STR technologies while mitigating their individual limitations [9] [1]. This comparative analysis examines the technical specifications, performance characteristics, and applications of DIP panels versus traditional STR forensic markers within population genetics research, with particular emphasis on their utility for analyzing allele frequency diversity across global populations.
Marker Technology and Mechanism Comparison
Fundamental Genetic Architectures
Short Tandem Repeats (STRs) are regions of the genome consisting of short, repetitive sequence elements (typically 2-6 base pairs) repeated in tandem [1]. The high polymorphism of STRs stems from variation in the number of repeat units, resulting in multiple alleles that can be separated and detected using capillary electrophoresis. The forensic community has standardized core STR loci that provide high discrimination power for individual identification, with commercial kits simultaneously analyzing 20 or more markers [2]. However, STR analysis generates stutter peaks—artifactual signals typically one repeat unit shorter than the true allele—which complicate mixture interpretation, particularly when the minor contributor represents less than 10% of the total DNA [16].
Deletion/Insertion Polymorphisms (DIPs), also known as indels, represent another class of length polymorphisms characterized by the presence or absence of specific DNA sequences at particular genomic locations [7]. These biallelic markers (typically featuring "insertion" [L] or "deletion" [S] alleles) offer several analytical advantages: they lack stutter artifacts, have lower mutation rates than STRs, and can be amplified in shorter amplicons suitable for degraded DNA analysis [16] [17]. DIPs combine desirable properties of both STRs and Single Nucleotide Polymorphisms (SNPs), with the abundance and stability of SNPs and the length-based detection simplicity of STRs [17].
The DIP-STR marker system represents an innovative compound approach that pairs a DIP with a closely linked STR polymorphism [4]. This configuration creates highly polymorphic haplotypes that can be targeted using allele-specific PCR primers. The strategic primer design enables selective amplification of the minor contributor's DNA in unbalanced mixtures by exploiting DIP genotype mismatches between contributors [9]. Specifically, when the major and minor contributors have opposite DIP homozygous genotypes (SS/LL or LL/SS) or when the major contributor is homozygous (SS or LL) and the minor contributor is heterozygous (SL), the DIP-STR system can preferentially amplify the minor component, effectively overcoming the masking effect that plagues conventional STR analysis [1].
Technical Workflow and Genotyping Mechanisms
The following diagram illustrates the conceptual framework for selecting appropriate forensic markers based on mixture characteristics and analytical requirements:
Performance Metrics and Comparative Analysis
Sensitivity and Mixture Deconvolution Capabilities
The capacity to detect minor DNA components in mixed samples represents a critical performance metric distinguishing these marker systems. Conventional STR markers typically require the minor contributor to represent at least 10-20% of the total DNA to generate a detectable profile [4] [2]. Below this threshold, the major contributor's alleles mask the minor component, rendering it undetectable through standard electrophoregram interpretation.
In contrast, DIP-STR markers demonstrate remarkable sensitivity, successfully genotyping minor components present at ratios as low as 1:1,000 (0.1%) [4] [9]. This 100-fold improvement in sensitivity stems from the allele-specific priming strategy that preferentially amplifies the minor contributor's DNA when DIP genotype mismatches exist between contributors [1]. This exceptional sensitivity enables applications impossible with standard STRs, including noninvasive prenatal paternity testing from maternal plasma, where fetal DNA constitutes only 3-20% of the total cell-free DNA [18].
DIP panels alone offer intermediate performance, with studies demonstrating reliable detection of minor components at ratios up to 1:40 [1]. While less sensitive than DIP-STRs for mixture deconvolution, DIPs provide advantages for analyzing degraded samples due to their shorter amplicon sizes and absence of stutter artifacts [16].
Forensic Efficiency Parameters and Population Genetics Applications
The following table compares key forensic efficiency parameters and population genetics applications across marker types:
Table 1: Comparative Analysis of Forensic Efficiency Parameters Across Marker Systems
| Parameter | STR Markers | DIP Panels | DIP-STR Markers |
|---|---|---|---|
| Typical Combined Discrimination Power | >0.999999999 (with 20+ loci) | 0.9999999999989 (30 loci in Kyrgyz) [17] | Highly polymorphic haplotypes provide similar discrimination to STRs [4] |
| Typical Combined Probability of Exclusion | >0.9999 (with 20+ loci) | 0.9939 (30 loci in Kyrgyz) [17] | Not specifically reported but expected to be high due to compound nature |
| Mutation Rate | ~10⁻³ - 10⁻⁴ (relatively high) | ~10⁻⁸ (very low) [7] | STR component has higher mutation rate than DIP component |
| Stutter Artifacts | Significant issue for mixture interpretation | None [16] | Reduced compared to STRs alone [9] |
| Ancestry Inference Capability | Limited with standard forensic panels | Strong with ancestry-informed DIP panels [7] [17] | Demonstrated for five major population groups [9] |
| Population Genetic Structure Resolution | Moderate | High for continental populations [17] | Comparable to small-scale ancestry informative markers [9] |
Analysis of Degraded DNA and Challenging Samples
The performance differential between marker systems becomes particularly pronounced when analyzing compromised forensic evidence. DIP markers consistently outperform STRs in degraded DNA analysis due to their shorter amplicon sizes [16]. Modern DIP panels specifically design amplicons under 200bp to maximize success with degraded templates [7], whereas standard STR amplification typically requires longer products (300-400bp). This size advantage enables more complete profiles from environmentally damaged or ancient DNA samples.
DIP-STR markers maintain this advantage, with validated assays averaging 230bp in length [9]. The combination of shorter amplicons with reduced PCR artifacts makes DIP-STRs particularly effective for "touch" DNA evidence, which often contains minimal template DNA in various states of degradation [9]. Standard STR systems frequently yield partial or unbalanced profiles from such challenging samples, while DIP-STRs can generate more complete genetic information from the same DNA extract.
Population Genetic Diversity and Allele Frequency Distribution
Global Population Studies and Genetic Structure Analyses
Comprehensive population genetic studies provide critical data for both forensic statistics and understanding human diversity. Research across global populations reveals distinct patterns of genetic variation accessible through different marker types. A study of 30 DIP loci in the Kyrgyz population from China's Xinjiang Uygur Autonomous Region demonstrated clear genetic relationships with other Central Asian groups (Kazakh and Uygur), while showing more distant relationships with East Asian, European, and Mexican Amerindian populations [17]. The combined power of discrimination for these 30 DIPs reached 0.9999999999989, sufficient for forensic identification purposes [17].
Recent investigations of 10 DIP-STR markers across four major U.S. population cohorts (European-American, African-American, Hispanic, and Asian-American) revealed fine-scale population substructure that previously escaped detection by more conventional marker systems [13]. The differential distribution of DIP-STR alleles among these populations reflected historical migrations, demographic events, and admixture patterns characteristic of the United States, with some markers showing strong ethnicity-specific allelic signatures [13].
The following diagram illustrates the experimental workflow for population genetic studies using DIP and DIP-STR markers:
Ancestry Inference and Biogeographical Applications
The selection of appropriate markers significantly impacts the resolution of ancestry inference in forensic and population genetic contexts. DIP panels specifically designed for ancestry information (AIM-DIPs) demonstrate remarkable utility in distinguishing geographically separated populations [7]. A 60-Plex DIP panel developed for East Asian populations successfully differentiated northern and southern East Asian subgroups, with principal component analysis, STRUCTURE analysis, and phylogenetic reconstructions consistently revealing population affinities that aligned with known historical migration patterns [7].
Similarly, a self-developed 43 autosomal DIP panel demonstrated strong performance in ancestry inference for Chinese Yi, Hani, and Miao groups from Yunnan Province, with machine learning algorithms (XGBoost and SVM) correctly classifying 82.39% of individuals to their continental origins [16]. The cumulative match probabilities for these groups reached extremely low values (10⁻¹⁸ to 10⁻¹⁹), demonstrating the utility of DIP panels for both identification and ancestry assessment [16].
DIP-STR markers have also proven valuable for biogeographic ancestry inference, with studies of 23 validated markers showing performance comparable to currently used small-scale ancestry informative markers for distinguishing five major population groups [9]. The compound nature of DIP-STRs provides both the stability of DIPs and the high polymorphism of STRs, creating informative haplotypes that reflect population history while maintaining forensic discrimination power.
Experimental Protocols and Methodologies
Standardized Laboratory Workflows
The laboratory procedures for generating population data follow standardized protocols optimized for each marker type. For DIP panel analysis, the typical workflow begins with DNA extraction from blood or buccal samples, followed by quantification to ensure optimal template concentration (typically 1-2 ng/μL) [7]. Multiplex PCR amplification employs specifically designed primer mixes targeting 30-60 DIP loci simultaneously, with careful optimization of primer concentrations to ensure balanced amplification [16]. Thermal cycling conditions typically include an initial denaturation at 94-95°C, followed by 25-30 cycles of denaturation, annealing (58-60°C), and extension, with a final extension step to ensure complete adenylation [7].
PCR products are separated by capillary electrophoresis using multi-color fluorescent detection systems, with internal size standards enabling precise fragment sizing [16]. Genotype calling utilizes specialized software (e.g., GeneMapper ID-X) with manual review to ensure accuracy, particularly for rare alleles. Following data generation, comprehensive quality control measures include testing for Hardy-Weinberg equilibrium, linkage disequilibrium, and stutter artifact assessment [17].
For DIP-STR analysis, the fundamental workflow remains similar but incorporates allele-specific primers that target either the insertion or deletion allele at each locus [4]. This requires additional validation to ensure primer specificity and optimal amplification conditions for the minor component in mixtures. Sensitivity testing establishes the limits of detection for minor contributors, with demonstrated capability to detect 0.1% minor components in artificial mixtures [9].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Research Reagent Solutions for Population Genetic Studies
| Reagent/Material | Function | Application Notes |
|---|---|---|
| Qiagen Investigator DIPplex Kit | Commercial multiplex PCR system for 30 DIP loci | Standardized protocol for population studies; used in Kyrgyz research [17] |
| AGCU DIP 50/60 Kits | Commercial DIP panels for forensic identification | Designed for Chinese populations; amplicons <200bp [16] |
| Self-Developed DIP Panels | Custom multiplex systems for specific research questions | Enable targeting of specific populations or research objectives [7] [16] |
| DIP-STR Primer Mixes | Allele-specific amplification of compound markers | Enable minor component detection in unbalanced mixtures [9] |
| ABI 3500xL Genetic Analyzer | Capillary electrophoresis for fragment separation | Standard platform for forensic genetics; multi-color detection [16] |
| GeneMapper ID-X Software | Genotype calling and allele designation | Automated analysis with manual review; essential for accurate population data [16] |
| STRAF (STR Analysis for Forensics) | Online tool for forensic parameters | Calculates allele frequencies, HWE, forensic efficiency metrics [16] |
The comparative analysis of DIP panels versus STR forensic markers reveals a complex landscape where marker selection must align with specific research objectives and sample characteristics. STR markers remain the gold standard for routine forensic identification where DNA quality is sufficient and mixtures are not extremely unbalanced. However, DIP panels offer significant advantages for ancestry inference, analysis of degraded DNA, and populations with established DIP databases. The absence of stutter artifacts, lower mutation rates, and shorter amplicon sizes make DIPs particularly suitable for challenging forensic evidence and population genetic studies requiring stable genetic markers.
The innovative DIP-STR compound markers represent a specialized solution for the most challenging forensic scenarios—extremely unbalanced mixtures regardless of contributor sexes. Their demonstrated ability to detect minor components at 1:1000 ratios exceeds the capabilities of both standard STRs and Y-STRs, while their compound nature provides high discrimination power suitable for identification purposes. As population databases for DIP-STRs expand, their utility in both forensic and population genetic contexts will continue to grow.
For researchers investigating allele frequency diversity across global cohorts, the strategic integration of multiple marker types—using DIPs for ancestry inference and stable population relationships, STRs for high-resolution identification, and DIP-STRs for specialized mixture analysis—provides the most comprehensive approach to understanding human genetic variation and its applications in both forensic and anthropological contexts.
In forensic genetics, the choice of genetic markers is paramount, influencing the power to identify individuals, deconvolve mixed samples, and infer biogeographical ancestry. This comparison guide focuses on two distinct types of markers: Deletion-Insertion Polymorphisms (DIPs) and Short Tandem Repeats (STRs). Their core difference lies in their mutational behavior; STRs are characterized by their hypervariability due to high mutation rates, while DIPs exhibit inherent stability with significantly lower mutation rates [4] [19]. This divergence stems from their fundamental molecular structures: STRs consist of tandemly repeated nucleotide units (e.g., "GAAA") that are prone to replication slippage, whereas DIPs are typically binary insertions or deletions of a short DNA sequence that are more stable [4] [20]. Understanding this contrast is critical for selecting the appropriate marker type for specific applications, from traditional human identification to analyzing highly unbalanced DNA mixtures.
Quantitative Data Comparison: DIPs vs. STRs
The following tables summarize key quantitative differences between DIP and STR markers, synthesizing data from foundational and recent studies.
Table 1: Core Property Comparison of DIP and STR Markers
| Property | DIPs (Deletion-Insertion Polymorphisms) | STRs (Short Tandem Repeats) |
|---|---|---|
| Molecular Nature | Binary insertion/deletion of a DNA sequence [4] | Tandemly repeated nucleotide units (e.g., di-, tri-, tetra-nucleotide repeats) [20] |
| Primary Mutation Mechanism | Replication error | Polymerase slippage during replication [20] |
| Typical Allele Designation | Presence (Ins) or Absence (Del) of sequence [4] | Number of repeat units, inferred from fragment length [20] |
| Inherent Mutation Rate | Low [4] [19] | Very High ("hypervariable") [21] [22] |
| Best-suited Applications | Minor component analysis in unbalanced mixtures, ancestry inference [4] [19] [13] | Human identity testing, paternity analysis, chimerism monitoring [23] [20] |
Table 2: Performance Metrics in Forensic-Type Analyses
| Metric | DIP-STR Compound Markers | Standard STRs |
|---|---|---|
| Sensitivity in Unbalanced Mixtures | Can genotype minor component at ratios up to 1:1,000 [4] | Minor component typically requires ≥20% of total DNA for unambiguous allele identification [4] |
| Multiplex Capability (MPS Panels) | Panels of 10+ markers used for ancestry and mixture deconvolution [19] [13] | Large panels available (e.g., 27 autosomal STRs amplified in a single reaction) [23] |
| Profile Recovery from Low DNA | Robust results reported with down to 25 pg of input DNA for some DIP-STR panels [23] | Performance varies; MPS-based STR kits typically require 100-125 pg of input DNA [23] |
| Power of Discrimination | High due to compound haplotypes (DIP + STR) [4] [13] | High due to multi-allelic nature and high heterozygosity [21] |
Experimental Data & Validation Studies
Establishing STR Hypervariability
The hypervariability of STRs is well-documented. A landmark study on Cannabis sativa demonstrated the power of just five STR markers to distinguish 89 out of 93 individual plants, detecting 79 distinct alleles across these loci [21]. This highlights the immense polymorphism and informativeness of STRs, which is directly tied to their high mutation rate. In human diagnostics, such as chimerism monitoring after stem cell transplantation, this variability is exploited to find donor-recipient pairs with informative allele size differences [20].
Recent human pedigree studies provide direct evidence for this hypervariability. A 2024 multigenerational, telomere-to-telomere sequencing study identified short tandem repeats and variable-number tandem repeats as "the most mutable" form of genetic variation, with 32 specific loci observed to undergo recurrent mutation across the generations [22].
Demonstrating DIP Stability and Utility in Mixtures
The stability of DIPs is leveraged in novel compound markers, such as DIP-STRs, which were specifically designed to analyze extremely unbalanced two-person DNA mixtures [4]. The underlying principle is that the stable, biallelic DIP allele is used to target the amplification of a closely linked, more variable STR in a single molecule. This design allows for the selective amplification of the minor contributor's DNA, even when it is masked by a vast excess of another DNA source.
Initial validation of an early set of nine DIP-STR markers demonstrated their ability to genotype a minor DNA component at ratios as extreme as 1:1,000 [4]. This performance starkly contrasts with the 1:5 ratio limit of standard STR analysis, highlighting how pairing a stable DIP with a variable STR overcomes the PCR amplification bias that plagues traditional markers in mixture contexts.
Detailed Experimental Protocols
Protocol for DIP-STR Analysis of Unbalanced Mixtures
This protocol is adapted from the pioneering work that developed the DIP-STR approach for targeting a minor donor in imbalanced DNA mixtures [4].
- Step 1: Marker Selection. Select a panel of DIP-STR markers where the DIP (e.g., a deletion) and the neighboring STR are in tight linkage. Markers should be located on different autosomal chromosomes to ensure independent inheritance and maximize discriminatory power.
- Step 2: PCR Amplification. Perform a single PCR reaction using primers that flank the compound DIP-STR locus. The reverse primer is designed to bind to the DIP allele (e.g., the inserted sequence) that is specific to the minor contributor. This ensures that amplification is preferentially initiated from the minor contributor's DNA template, even in the presence of a vast excess of the major contributor's DNA.
- Step 3: Fragment Analysis or Sequencing. Analyze the PCR products using capillary electrophoresis for fragment analysis or massively parallel sequencing (MPS). The output will be the STR allele(s) linked to the specific, targeted DIP allele.
- Step 4: Data Interpretation. The generated STR profile is unequivocally assigned to the minor contributor in the mixture. The combined DIP-STR haplotype provides a highly specific genetic signature for identity testing.
Protocol for MPS-Based STR Profiling with Mixture Detection
This protocol outlines the standard workflow for forensic STR analysis using Massively Parallel Sequencing, which can handle moderate mixtures and reveal sequence-level STR variation [23].
- Step 1: Library Preparation. Extract genomic DNA from the sample. Use a commercial forensic MPS kit, such as the ForenSeq DNA Signature Prep Kit. This involves a multiplex PCR amplification that simultaneously targets dozens of STRs (autosomal, X, Y) and hundreds of Single Nucleotide Polymorphisms (SNPs). The PCR adds unique adapter sequences to each amplified fragment.
- Step 2: Sequencing. Pool the purified amplified libraries and load them onto a sequencing platform, such as the MiSeq FGx. Sequencing-by-synthesis is performed to generate millions of short reads.
- Step 3: Data Analysis. Process the raw data using specialized software (e.g., ForenSeq Universal Analysis Software). The software aligns the sequences to a reference genome, calls alleles for each STR and SNP, and flags the potential presence of a mixture based on the detection of more than two alleles at multiple loci.
- Step 4: Mixture Deconvolution. For mixtures, the analyst uses the software's output and statistical models to separate the contributing genotypes, a process that is more effective with the sequence-level detail provided by MPS compared to traditional capillary electrophoresis.
Visualization of Workflows and Mutational Mechanisms
The following diagram illustrates the core conceptual difference between the mutational mechanisms of STRs and DIPs, which underpins their respective hypervariability and stability.
The Scientist's Toolkit: Essential Research Reagents & Materials
Table 3: Key Research Reagents and Solutions for DIP and STR Analysis
| Reagent / Solution | Function / Description | Example Use-Case |
|---|---|---|
| ForenSeq DNA Signature Prep Kit (Verogen) | Multiplex PCR primer mix for simultaneous amplification of STRs, SNPs, and identity markers for MPS [23] | Comprehensive human identification and phenotyping from forensic samples. |
| Precision ID GlobalFiler NGS STR Panel (Thermo Fisher) | Multiplex assay targeting 35 STR loci, including the 21 CODIS markers, optimized for degraded DNA [23] | STR profiling for database entry and mixture deconvolution using an Ion GeneStudio S5 system. |
| Precision ID Ancestry Panel (Thermo Fisher) | Targets 165 autosomal SNPs for biogeographical ancestry (BGA) inference [23] | Inference of the biogeographical origin of a sample contributor. |
| DIP-STR Specific Primers | Custom-designed PCR primers that bind to a specific DIP allele and flank the adjacent STR [4] | Selective amplification of a minor DNA component in a highly unbalanced mixture (e.g., 1:1000). |
| MiSeq FGx Forensic Genomics System (Verogen) | Integrated platform including sequencer, reagents, and analysis software for forensic genomics [23] | End-to-end workflow for MPS-based STR and SNP sequencing in a forensically validated environment. |
| Converge Software (Thermo Fisher) | NGS data analysis module for processing data from Precision ID panels on Ion Torrent systems [23] | Genotype calling and analysis for STR and SNP panels. |
The comparative analysis between DIPs and STRs reveals a clear dichotomy defined by mutation rate: STR hypervariability versus DIP stability. STRs, with their high mutation rate and multi-allelic nature, remain the gold standard for direct human identification and paternity testing where sample quality is sufficient. However, the inherent stability of DIPs, particularly when haplotyped with nearby STRs in DIP-STR markers, provides a powerful tool for tackling complex forensic challenges, most notably the genotyping of a minor contributor in severely unbalanced DNA mixtures. The choice between these markers is not a matter of superiority but of application-specific suitability. As forensic science evolves, MPS technologies are increasingly enabling the simultaneous analysis of both stable and hypervariable markers, offering a more complete genetic picture for advanced forensic investigations [23] [19] [13].
Practical Deployment: Forensic Applications and Workflow Integration
Short Tandem Repeat (STR) analysis via capillary electrophoresis (CE) represents the cornerstone of modern forensic DNA profiling, providing robust and reliable results for the vast majority of casework samples. However, the analysis of challenging DNA mixtures, particularly those with highly unbalanced contributor ratios, remains a significant limitation of standard STR protocols. This comparative guide examines the performance of standard STR markers against a novel class of compound markers, DIP-STRs, which were specifically developed to deconvolute mixtures where the minor contributor constitutes less than 10% of the total DNA. We will objectively compare these systems based on experimental data, detailing their protocols, sensitivities, and appropriate forensic applications to guide researchers and scientists in selecting the optimal method for their analytical challenges.
Performance Comparison: STR vs. DIP-STR Markers
The primary challenge in forensic DNA mixture analysis arises when the contributions from two or more individuals are highly unbalanced. Standard STR markers, while excellent for single-source or moderately mixed samples, encounter a fundamental detection limit. As extensively documented, the minor contributor in a mixed DNA stain cannot be successfully detected using STRs if its share is less than 10% of the total DNA, as the major contributor's profile effectively "masks" that of the minor contributor [2]. This limitation impacts cases ranging from sexual assaults, where the victim's DNA is predominant, to the analysis of "touch" DNA evidence [2] [24].
In contrast, DIP-STR markers are a compound marker system designed to overcome this imbalance. They consist of a Deletion/Insertion Polymorphism (DIP) linked closely to a Short Tandem Repeat (STR) polymorphism [4]. The power of this system lies in its allele-specific design. By using primers that target the DIP allele unique to the minor contributor, the DIP-STR method can selectively amplify the minor DNA component, even in the presence of a massive excess of the major contributor's DNA [24]. Experimental data demonstrates that DIP-STRs can genotype the minor component at ratios as extreme as 1:1,000 (0.1%), far surpassing the capabilities of standard STRs [9] [4] [24].
The table below summarizes the key performance characteristics of the two systems based on published experimental findings:
Table 1: Experimental Performance Comparison of STR and DIP-STR Markers
| Performance Characteristic | Standard STR Markers | DIP-STR Markers |
|---|---|---|
| Effective Minor Contributor Detection Limit | ~10% (1:10 ratio) [2] | ~0.1% (1:1000 ratio) [9] [4] |
| Typical Application Scenario | Moderately unbalanced mixtures [2] | Extremely unbalanced mixtures [2] |
| Dependence on Sex of Contributors | No | No |
| Application in "Touch" DNA Casework | Limited in highly unbalanced mixes | Effective detection in 54 out of 71 simulated traces [24] |
| Multiplexing Potential | High (e.g., PowerPlex Fusion 6-dye system) [25] | Growing (e.g., validated panels of 10, 23, and 30 markers) [9] [6] |
| Primary Forensic Advantage | Gold standard for most routine casework; high discrimination power | Unmatched sensitivity for minor DNA in extreme ratio mixtures |
A critical application is the analysis of "touch" DNA, where the amount of DNA recovered is often low and mixtures are common. One study simulating 71 unbalanced contact traces found that DIP-STRs detected the minor DNA contributor in 54 out of 71 traces, irrespective of sex. Y-STRs, a common alternative for male-minor/female-major mixtures, were only applicable to 14 of the traces and showed comparable sensitivity only within that subset [24]. This underscores the DIP-STR's utility in a wider range of scenarios.
Experimental Protocols and Workflows
Standard STR Analysis via Capillary Electrophoresis
The protocol for standard STR analysis is a well-established and highly standardized process in forensic laboratories. The following workflow, consistent with protocols from the New York City Office of Chief Medical Examiner, outlines the core steps [25].
Figure 1: Standard STR Analysis Workflow
- DNA Extraction: This initial step involves isolating DNA from biological material. Protocols are tailored to the sample type, such as differential extraction for semen stains/swabs, organic extraction, or use of automated systems like the EZ1 Advanced XL or MaxSuite with DNA IQ beads [25].
- DNA Quantitation: The quantity of recovered DNA is accurately measured using kits such as the Quantifiler Trio DNA Quantification Kit to ensure optimal amplification in subsequent steps [25].
- PCR Amplification: Specific STR loci are amplified using commercially available kits like the PowerPlex Fusion System, which co-amplifies over 20 STR loci plus amelogenin for sex determination. This is typically performed on a thermal cycler such as the Mastercycler X50s [25].
- Capillary Electrophoresis (CE): The amplified DNA fragments are separated by size via CE on instruments like the 3500xL Genetic Analyzer. Internal size standards allow for precise allele calling [25].
- Analysis and Interpretation: The resulting electrophoretograms are analyzed using software such as GeneMarker HID. Profiles are interpreted according to laboratory guidelines, which for mixtures may involve probabilistic genotyping software like STRmix [25].
- Statistical Analysis: The statistical significance of a match is calculated using population frequency databases and specialized tools like the Forensic Statistical Tool [25].
DIP-STR Analysis for Unbalanced Mixtures
The DIP-STR protocol shares some steps with standard STR analysis but incorporates a crucial allele-specific amplification strategy. The workflow is designed to target the minor contributor's DNA selectively [4] [24].
Figure 2: DIP-STR Targeted Analysis Workflow
- Allele-Specific PCR Amplification: This is the key differentiator. The DIP-STR method uses PCR primers specifically designed to bind to the DIP allele (either the insertion or deletion) that is unique to the minor contributor. This design preferentially amplifies the DIP-STR haplotype of the minor DNA, even when it is vastly outnumbered by the major DNA [4]. A marker is considered "informative" when the major and minor contributors have different DIP alleles.
- Haplotype Analysis: The CE output provides a haplotype, which is the combined DIP and STR allele. The STR component remains highly polymorphic, providing the discrimination power needed for identification [4].
- Statistical Interpretation: The results are evaluated using an object-oriented Bayesian network, which calculates a likelihood ratio for the hypothesis that the minor contributor is a specific suspect versus an unknown, unrelated person [2].
The Scientist's Toolkit: Essential Reagents and Materials
Table 2: Key Research Reagent Solutions for STR and DIP-STR Analysis
| Item | Function in Protocol | Specific Examples / Kits |
|---|---|---|
| DNA Extraction Kits | Isolation of pure DNA from complex biological samples. | QIAcube kits, EZ1 Advanced XL, DNA IQ beads (MaxSuite) [25]. |
| Quantitation Kits | Precise measurement of DNA concentration to ensure optimal PCR. | Quantifiler Trio DNA Quantification Kit [25]. |
| STR Amplification Kits | Multiplex PCR amplification of core STR loci. | PowerPlex Fusion System, PowerPlex Y23 System [25]. |
| DIP-STR Primer Panels | Allele-specific amplification of target DIP-STR haplotypes. | Validated panels of 10, 23, or 30 DIP-STR markers [9] [24]. |
| Thermal Cyclers | Performing precise PCR temperature cycles for DNA amplification. | Mastercycler X50s [25]. |
| Capillary Electrophoresis Instruments | High-resolution separation of amplified DNA fragments by size. | 3500xL Genetic Analyzer [25]. |
| Size Standards & Ladders | Accurate sizing of DNA fragments during CE analysis. | PowerPlex Fusion Ladder and Size Standard [25]. |
| Probabilistic Genotyping Software | Interpreting complex DNA mixtures and calculating statistical weight of evidence. | STRmix [25]. |
STR analysis via capillary electrophoresis remains the undisputed gold standard for routine forensic casework, offering robust, reliable, and highly discriminating results for most samples. However, for the critical challenge of extremely unbalanced DNA mixtures—where the minor contributor represents less than 1% of the total DNA—DIP-STR markers offer a transformative solution. Experimental data consistently shows that DIP-STRs can successfully genotype minor contributors at ratios as low as 1:1000, a sensitivity an order of magnitude greater than standard STRs. The choice between these systems is not one of replacement but of appropriate application. Standard STR protocols are the first and best choice for the majority of casework. In contrast, DIP-STRs represent a powerful, specialized tool for the most challenging mixed stains, enabling DNA profiling in cases previously deemed intractable and thereby expanding the frontiers of forensic genetic analysis.
Forensic genetics consistently faces the challenge of analyzing complex biological samples containing DNA from multiple contributors in highly unbalanced ratios. Traditional Short Tandem Repeat (STR) markers, the gold standard in forensic human identification, encounter significant limitations when the minor contributor represents less than 5-10% of the total DNA mixture [24]. This technological gap has driven the development of innovative genetic markers, notably Deletion/Insertion Polymorphisms (DIPs) and the compound marker DIP-STRs, which offer enhanced sensitivity for targeting minor DNA components in unbalanced mixtures.
DIPs, also known as InDels, are bi-allelic length polymorphisms characterized by the presence or absence of short DNA sequences. Their key forensic advantages include low mutation rates, absence of stutter peaks during amplification, and short amplicon sizes ideal for analyzing degraded DNA [10] [16]. DIP-STRs represent a more sophisticated approach, combining a slow-evolving DIP with a closely linked, fast-evolving STR to create a highly informative compound haplotype [19] [4]. This guide provides a comparative analysis of these marker systems against traditional STRs, supported by experimental data and methodological protocols.
Marker Comparison: Technical Specifications and Performance Metrics
The table below summarizes the core characteristics and forensic performance of STR, DIP, and DIP-STR marker systems.
Table 1: Comparative Analysis of Forensic Genetic Markers
| Feature | STR Markers | DIP Panels | DIP-STR Markers |
|---|---|---|---|
| Molecular Nature | Multi-allelic, length polymorphism (tandem repeats) | Bi-allelic, length polymorphism (insertion/deletion) | Compound marker (DIP + closely linked STR) |
| Mutation Rate | High (~10⁻³) | Low (~10⁻⁸) [7] | Combination of low (DIP) and high (STR) rates |
| Stutter Peaks | Significant issue | None [16] | Minimal (from STR component) |
| Typing Platform | Capillary Electrophoresis (CE) | Capillary Electrophoresis (CE) [7] | CE or Next-Generation Sequencing (NGS) [12] |
| Detection Limit in Mixtures | 1:10 to 1:20 (Minor:Major) | Varies with panel | Up to 1:1000 (Minor:Major) [24] [4] |
| Primary Forensic Application | Individual identification, DNA databases | Individual identification, ancestry inference [10] [16] | Deconvolution of unbalanced mixtures, minor contributor identification [19] [24] |
| Example Kits/Panels | AmpFℓSTR NGMSElect, PowerPlex ESI17 [26] | Investigator DIPplex, 60-Panel (56 A-DIPs) [10] [7] | 10-plex DIP-STR panel [19], 6-plex DIP-STR panel [24] |
Experimental Workflows: From Sample to Profile
The successful application of DIP and DIP-STR markers relies on robust and validated experimental protocols. The following diagram and sections detail the standard workflow.
Diagram 1: DIP and DIP-STR Analysis Workflow
DNA Extraction and Multiplex PCR Amplification
The process begins with standard DNA extraction from forensic samples. For DIP and DIP-STR analysis, the subsequent multiplex PCR is critical.
DIP Panel Protocol: A 60-plex DIP panel protocol uses a 6-dye system to simultaneously type 56 autosomal DIPs, 3 Y-chromosome DIPs, and the Amelogenin locus for sex determination. The reaction undergoes amplification on a thermal cycler with carefully optimized conditions: an initial denaturation at 94°C, followed by 25 cycles of denaturation (94°C), annealing (60°C), and extension (72°C), with a final extension at 60°C for 25 minutes [7]. The amplicons are kept short (under 200 bp) to facilitate the analysis of degraded DNA.
DIP-STR Protocol: The PCR exploits allele-specific amplification primed by the DIP allele. A primer is designed to bind specifically to one DIP allele (e.g., the insertion). In a mixture, if the minor contributor possesses a unique DIP allele not present in the major donor, the primer will selectively amplify the linked STR from the minor contributor's DNA, thereby bypassing the masking effect of the major DNA [24] [4].
Genotyping and Data Interpretation
Following amplification, products are separated and detected by Capillary Electrophoresis (CE), similar to standard STR typing. The resulting electropherograms are analyzed with software like GeneMapper ID-X.
DIP Analysis: Genotyping is straightforward due to the bi-allelic nature of DIPs. Each locus shows one or two peaks (for heterozygotes), and the absence of stutter simplifies analysis [16]. Population genetic parameters (e.g., Hardy-Weinberg Equilibrium, Linkage Disequilibrium) and forensic efficiency parameters (e.g., Power of Discrimination, Probability of Exclusion) are then calculated [10] [16].
DIP-STR Analysis: The result is a haplotype combining the DIP state and the STR repeat number. The analysis focuses on detecting the minor contributor's unique haplotypes. For investigative leads, the haplotype can be searched against population databases. Statistical weight is evaluated using specialized Bayesian network models to account for the compound marker's inheritance [24].
Performance Data: Sensitivity, Mixture Deconvolution, and Ancestry Inference
Sensitivity in Unbalanced Mixtures
The paramount advantage of DIP-STR markers is their exceptional performance in extremely unbalanced mixtures.
Simulated "Touch" DNA Traces: In a study analyzing 71 simulated two-source contact traces, a set of six DIP-STRs was compared to Y-STRs. In traces with a male minor contributor, DIP-STRs showed similar sensitivity to Y-STRs. Crucially, DIP-STRs were also effective in 57 traces where Y-STRs were inapplicable (e.g., female-minor mixtures), successfully detecting the minor contributor in 30 of them [24]. This demonstrates the marker's independence from the sex of the contributors.
Controlled Mixture Experiments: The DIP-STR method has been proven to reliably genotype the minor component in a DNA mixture at ratios as extreme as 1:1,000 (minor:major), far exceeding the capabilities of standard STRs [4].
Forensic Efficiency for Identification and Ancestry
While DIP-STRs excel in mixture deconvolution, larger DIP panels are highly effective for individual identification and biogeographical ancestry inference.
Table 2: Forensic Efficiency Metrics of DIP Panels in Various Populations
| Population Group | Panel Used | Combined Power of Discrimination (CPD) | Cumulative Probability of Exclusion (CPE) | Ancestry Inference Capability |
|---|---|---|---|---|
| East Asian (General) | 60-plex DIP Panel | 0.999999999999 | 0.9937 [10] [7] | Effective for East Asian subgroups [7] |
| Chinese Yunnan Groups (Yi, Hani, Miao) | 43 A-DIP Panel | ~1.11 x 10⁻¹⁸ (CMP*) | 0.9996 [16] | Close affinity with other East Asian populations [16] |
| Four US Populations (African, European, East Asian, Hispanic) | 10 DIP-STR Markers | N/A | N/A | Effectively distinguished the four major US groups [19] [13] |
CMP: Cumulative Match Probability
The 10 DIP-STR markers tested across four US populations revealed 116 unique haplotypes, with 44.8% present across groups and others being population-specific, providing valuable ancestry information [19] [13].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for DIP/DIP-STR Research
| Item | Function/Description | Example/Specification |
|---|---|---|
| Multiplex PCR Assay | Core reagent for simultaneous amplification of multiple markers. | Self-developed 43 A-DIP panel [16]; 60-plex DIP panel [7] |
| Thermal Cycler | Instrument for performing precise PCR amplification. | 9700 Thermal Cycler (Applied Biosystems) [16] |
| Genetic Analyzer | Capillary electrophoresis system for fragment size separation. | ABI 3500xL Genetic Analyzer [16] |
| Internal Size Standard | Essential for accurate fragment sizing in CE. | ORG 500 (Microread Genetics) [16] |
| Genotyping Software | Software for automated allele calling from CE data. | GeneMapper ID-X Software v1.5 [16] |
| Population Databases | Reference data for calculating allele frequencies and ancestry inference. | 1000 Genomes Project; HGDP-CEPH samples [19] [7] |
The comparative analysis confirms that DIP-based panels and DIP-STR markers significantly extend the capabilities of forensic genetics beyond the limits of traditional STRs. While STRs remain the cornerstone for database matching and standard individual identification, DIP panels offer a robust solution for personal identification and ancestry inference, particularly with degraded DNA. The DIP-STR system, in turn, represents a specialized, powerful tool for resolving the most challenging unbalanced DNA mixtures, enabling the detection of a minor contributor at ratios as low as 1:1000. The choice between these systems should be guided by the specific context of the forensic sample—with DIP-STRs being the superior option for extracting a single profile from a complex, imbalanced mixture.
The field of forensic genetics is undergoing a significant transformation with the adoption of sophisticated marker systems for biogeographic ancestry inference. While Short Tandem Repeats (STRs) have long been the workhorse of forensic human identification, Deletion/Insertion Polymorphisms (DIPs) are emerging as powerful tools for deciphering population origins. These bi-allelic markers, characterized by their low mutation rates and absence of stutter artifacts, provide enhanced capabilities for analyzing challenging forensic samples, including degraded DNA and unbalanced mixtures [6]. This comparative analysis examines the performance of DIP-based panels against conventional STR markers for ancestry inference, highlighting methodological advantages, experimental validations, and practical applications within forensic science.
The limitations of conventional STR systems have become increasingly apparent as forensic laboratories handle more complex casework. Standard STR kits, often optimized for specific populations, may show reduced discriminatory power in endogamous groups with distinct genetic structures [14]. Additionally, the larger amplicon sizes of traditional STR markers present challenges for degraded DNA analysis, frequently resulting in partial profiles and allele dropout [14]. These constraints have accelerated the development of DIP-based panels that offer improved resolution for ancestry assignment while maintaining robustness with suboptimal sample quality.
Comparative Analysis of DIPs and STRs as Forensic Markers
Fundamental Characteristics and Technical Advantages
DIPs, also known as insertion-deletion polymorphisms, represent a category of genetic variation characterized by the presence or absence of specific DNA sequences. Unlike multi-allelic STRs with their high mutation rates, DIPs are bi-allelic markers with mutation rates estimated on the order of 10⁻⁸, ensuring remarkable genomic stability over generations [6]. This fundamental difference in mutation rates translates to significant practical advantages for ancestry inference applications where population-specific allele frequencies must remain relatively stable over time.
From a technical standpoint, DIP analysis circumvents several analytical challenges inherent to STR profiling. The absence of stutter peaks during capillary electrophoresis analysis significantly improves typing accuracy and facilitates clearer interpretation of results [6]. Furthermore, DIP markers can be designed with shorter amplicons (typically under 200 bp) without sacrificing informational content, making them particularly suitable for analyzing degraded forensic evidence where DNA fragmentation has occurred [6].
Table 1: Comparative Characteristics of DIP and STR Markers for Forensic Analysis
| Characteristic | DIP Markers | STR Markers |
|---|---|---|
| Mutation Rate | ~10⁻⁸ (low) [6] | ~10⁻³ (high) [1] |
| Stutter Artifacts | Absent [6] | Present, complicating analysis [14] |
| Amplicon Size | Can be optimized <200 bp [6] | Typically larger, prone to dropout in degraded DNA [14] |
| Number of Alleles | Bi-allelic [6] | Multi-allelic [14] |
| Population Specificity | High for ancestry-informative DIPs [6] | Variable, often requires population-specific kits [14] |
| Typing Platform | CE or MPS [6] | Primarily CE [14] |
Compound Markers: DIP-STRs for Enhanced Mixture Deconvolution
A significant innovation in forensic genetics has been the development of compound markers that combine the advantages of different polymorphism types. DIP-STR markers link a deletion/insertion polymorphism with a closely associated short tandem repeat, creating highly polymorphic haplotypes that facilitate the analysis of extremely unbalanced DNA mixtures [1] [4]. These markers enable the targeting of minor DNA components in mixtures with ratios as extreme as 1:1000 (minor:major), far surpassing the capabilities of conventional STR analysis, which typically fails when the minor component represents less than 5-10% of the mixture [2] [4] [27].
The mechanism of DIP-STR analysis relies on allele-specific primers designed to target either the insertion (L allele) or deletion (S allele) variant of the DIP component. This design allows for selective amplification of the minor contributor's DNA despite overwhelming background DNA from the major contributor [1] [27]. The linked STR component then provides additional discrimination power through its polymorphic repeat region, creating a composite marker with enhanced sensitivity and specificity for mixture deconvolution [9].
Recent research has expanded the available DIP-STR markers through analysis of whole-genome sequencing data. Examination of the Genome Aggregation Database (gnomAD) comprising 76,156 genomes revealed hundreds of potential DIP-STR candidates throughout the genome [9]. Through stringent selection criteria and empirical validation, researchers have expanded forensic DIP-STR sets to 30 markers, providing the foundation for developing multiplex assays that improve success rates with trace DNA evidence [9].
DIP Panel Development and Validation for Ancestry Inference
Marker Selection and Panel Construction
The development of effective DIP panels for ancestry inference follows rigorous bioinformatic selection processes to identify markers with maximal population differentiation. A recent study established a 60-marker DIP panel specifically tailored for East Asian populations through systematic analysis of population genetic data from the 1000 Genomes Project and Nucleotide Polymorphism Database [6]. The selection criteria exemplified the stringency required for effective ancestry inference panels:
- Minimum allele frequency (MAF) ≥ 0.1 across populations to ensure sufficient variability
- Bi-allelic ins, del, or delins polymorphisms with length variations of 1-20 bp
- Physical separation on different chromosomes or >5 Mb apart on the same chromosomal arm to ensure independent inheritance
- Significant allele frequency differences between populations (pairwise differences ≥0.5 for African, European, and East Asian populations) [6]
This meticulous selection process yielded a panel comprising 56 autosomal DIPs, 3 Y-chromosome DIPs, and the Amelogenin sex-determination marker, with amplicons limited to 200 bp to enhance performance with degraded forensic samples [6].
Ancestry Inference Capabilities and Population Differentiation
The capacity of DIP panels to resolve fine-scale population structure has been demonstrated across multiple studies. The 60-plex DIP panel successfully differentiated northern and southern East Asian populations through principal component analysis (PCA), STRUCTURE analysis, and phylogenetic tree construction, with results consistent with historical migration patterns and known population relationships [6]. Similarly, research on DIP-STR markers revealed their utility as ancestry informative markers (AIMs), with a small set of 10 DIP-STRs effectively distinguishing four major U.S. population groups (African American, European American, East Asian American, and Southwest Hispanic) [19].
Table 2: Performance Metrics of DIP-Based Panels in Forensic Applications
| Panel Type | Population Studied | Key Performance Metrics | Reference |
|---|---|---|---|
| 60-plex DIP Panel | East Asian | CPD: 0.999999999999, CPE: 0.9937 | [6] |
| 10 DIP-STR Markers | US Population Groups | 44.8% haplotypes present across groups, unique haplotypes in specific populations | [19] |
| 7 DIP-STR Markers | Southwest Chinese Han | Heterozygosity >0.50, 5-16 haplotypes per locus | [27] |
| 23 Novel DIP-STRs | Swiss Population | Sensitive down to 0.06 ng DNA, minor contributor detection at 0.1% | [9] |
The forensic statistical parameters of the 60-plex DIP panel demonstrate remarkable discrimination power, with a combined probability of discrimination (CPD) of 0.999999999999 and cumulative probability of paternity exclusion (CPE) of 0.9937, indicating not only utility for ancestry inference but also for personal identification [6]. These values confirm that carefully selected DIP panels provide sufficient polymorphism for definitive forensic conclusions while offering biogeographic insights beyond the capabilities of standard STR profiling.
Experimental Protocols and Methodological Considerations
Developmental Validation of DIP Panels
The validation of DIP panels for forensic implementation follows established guidelines recommended by the Scientific Working Group on DNA Analysis Methods (SWGDAM) to ensure reliability and reproducibility [6]. Comprehensive validation studies assess multiple performance parameters:
- PCR conditions optimization, including annealing temperature, cycling numbers, and reagent concentrations
- Sensitivity determination through serial dilution studies
- Species specificity to confirm cross-reactivity limitations
- Stability testing with degraded and inhibited samples
- Mixture analysis to establish detection limits for minor contributors
- Reproducibility across multiple operators and instruments
- Case-type samples analysis to simulate forensic applications [6]
For the 60-plex DIP panel, validation studies confirmed reliable genotyping even with poor-quality samples, demonstrating particular effectiveness with degraded DNA – a common challenge in forensic casework [6]. The panel maintained robust performance across various testing conditions, supporting its implementation for routine forensic applications.
Analytical Workflow for DIP-Based Ancestry Inference
The following diagram illustrates the comprehensive workflow for DIP-based ancestry inference, from marker selection to final biogeographic assignment:
This workflow begins with comprehensive analysis of whole-genome sequencing data from diverse populations to identify DIP candidates with significant allele frequency differences across biogeographic groups [6] [9]. Following multiplex panel design and validation, sample genotyping data undergoes multiple computational analyses to determine population affiliations. Principal Component Analysis reduces dimensionality to visualize genetic relationships, STRUCTURE analysis estimates ancestral proportions, and phylogenetic tree construction reveals evolutionary relationships among populations [6]. This multi-analytical approach provides robust ancestry inference with statistical confidence measures.
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful implementation of DIP-based ancestry inference requires specific reagents and analytical tools optimized for this application. The following table summarizes key components of the research toolkit:
Table 3: Essential Research Reagents and Materials for DIP-Based Ancestry Inference
| Reagent/Material | Specifications | Function in Workflow |
|---|---|---|
| DIP Multiplex Panel | 56 autosomal DIPs, 3 Y-DIPs, Amelogenin [6] | Target amplification for ancestry-informative markers |
| Allele-Specific Primers | S-primer (deletion), L-primer (insertion) [27] | Selective amplification of DIP alleles in mixture deconvolution |
| Capillary Electrophoresis System | 6-dye detection system [6] | Fragment separation and detection |
| Population Reference Databases | 1000 Genomes Project, HGDP-CEPH [6] [19] | Reference data for ancestry assignment |
| Bioinformatic Tools | STRUCTURE, PCA algorithms, Phylogenetic tree software [6] | Population genetic analysis and visualization |
| Whole Genome Sequencing Data | gnomAD (76,156 genomes) [9] | Marker discovery and validation |
The selection of appropriate DIP markers represents a critical foundational step, with optimal candidates demonstrating high heterozygosity, low stutter formation, and significant allele frequency differentiation across target populations [6]. The 6-dye capillary electrophoresis system enables multiplexed analysis of dozens of markers simultaneously, improving efficiency while conserving precious forensic samples. Computational tools for population genetic analysis complete the toolkit, transforming raw genotyping data into interpretable ancestry inferences.
The integration of DIP-based panels into forensic practice represents a significant advancement in ancestry inference capabilities. The demonstrated superiority of DIP markers over conventional STRs in analyzing degraded DNA and resolving unbalanced mixtures positions this technology as a transformative tool for forensic geneticists [6] [4]. The combined advantages of low mutation rates, absence of stutter artifacts, short amplicon sizes, and high population differentiation establish DIP markers as powerful alternatives for challenging forensic specimens where traditional STR profiling reaches its limitations.
Future developments in DIP-based ancestry inference will likely focus on several key areas. The continued expansion of population reference databases will enhance assignment accuracy across global populations, particularly for admixed groups. The integration of DIP analysis with massively parallel sequencing platforms will enable higher multiplexing capacities and more efficient analysis of multiple marker types in single assays [28]. Furthermore, the combination of DIP markers with other compound marker systems, such as SNP-STRs, may provide enhanced discrimination power for both identity testing and ancestry inference [27]. As these technologies mature and validation studies expand, DIP-based panels are poised to become standard tools in forensic genetic laboratories worldwide, providing crucial biogeographic intelligence for investigative leads.
Forensic genetics relies on the analysis of polymorphic genetic markers to identify individuals from biological evidence. For decades, short tandem repeats (STRs) have served as the gold standard for forensic DNA profiling worldwide due to their high discrimination power [29]. However, the limitations of STRs in analyzing challenging samples, such as highly unbalanced DNA mixtures or degraded specimens, have prompted the development of alternative markers, including deletion/insertion polymorphisms (DIPs) [4] [6].
This guide provides a comparative analysis of DIP panels versus traditional STR markers, focusing on the core forensic parameters of combined discrimination power and paternity exclusion power. We present objective performance comparisons and supporting experimental data to assist researchers, forensic scientists, and DNA analysts in selecting appropriate markers for specific identification scenarios.
Marker Technologies: Principles and Workflows
Short Tandem Repeats (STRs)
STRs are regions of DNA consisting of short, repetitive sequence motifs (typically 2-6 base pairs) tandemly repeated. The number of repeats varies between individuals, creating length polymorphisms that can be separated and visualized using capillary electrophoresis [29]. Standard forensic STR analysis involves:
- Multiplex PCR amplification of multiple STR loci
- Capillary electrophoresis for fragment size separation
- Comparison with allelic ladders for genotype determination
STRs provide high discrimination power due to their multi-allelic nature, but they face limitations with unbalanced DNA mixtures where the minor contributor represents less than 10% of the total DNA [2].
Deletion/Insertion Polymorphisms (DIPs)
DIPs, also known as insertion/deletion (InDel) polymorphisms, are bi-allelic markers resulting from the presence or absence of a specific DNA sequence. While individually less informative than STRs due to their biallelic nature, DIPs offer advantages including:
- Lower mutation rates (approximately 10⁻⁸) than STRs
- Absence of stutter peaks during capillary electrophoresis
- Shorter amplicon sizes suitable for degraded DNA
- Simpler interpretation of mixed profiles [6] [1]
Compound markers such as DIP-STRs (which pair a DIP with a nearby STR) and Multi-InDels (closely linked DIPs forming a microhaplotype) have been developed to enhance polymorphism while maintaining the advantages of DIPs [4] [30].
Workflow Comparison: STRs vs. DIP-STRs
The diagram below illustrates the fundamental structural difference between a standard STR marker and a compound DIP-STR marker, which underlies their different applications in mixture deconvolution.
Quantitative Performance Comparison
Forensic Effectiveness Parameters
The performance of forensic markers is quantitatively evaluated using several key parameters:
- Power of Discrimination (PD): Probability that two randomly selected individuals have different genotypes
- Combined Power of Discrimination (CPD): Cumulative PD across multiple marker loci
- Power of Exclusion (PE): Probability of excluding a random individual as the biological parent
- Combined Power of Exclusion (CPE): Cumulative PE across multiple marker loci
- Polymorphism Information Content (PIC): Measure of a marker's informativeness
- Random Match Probability (RMP): Probability that two randomly selected individuals have matching genotypes [6] [29] [30]
Performance Data Comparison
Table 1: Forensic Effectiveness Comparison of Different Marker Systems
| Marker System | Population | Cumulative Power of Discrimination (CPD) | Cumulative Power of Exclusion (CPE) | Key Advantages | Reference |
|---|---|---|---|---|---|
| 15 STR Loci | Khuzestan Province, Iran | 0.999999999 (estimated) | 0.9999 (estimated) | High polymorphism, established databases | [29] |
| 60-Plex DIP Panel | East Asian | 0.999999999999 | 0.9937 | No stutter peaks, low mutation rate, works with degraded DNA | [6] |
| 41 Multi-InDel Panel | Chinese Kazakh | 0.999999999999999999999999835993 | 0.999998887418153 | Enhanced mixture detection, short amplicons | [30] |
| 41 Multi-InDel Panel | Chinese Kyrgyz | 0.999999999999999999999999717184 | 0.999999348634116 | Enhanced mixture detection, short amplicons | [30] |
Table 2: Performance in Unbalanced DNA Mixtures
| Marker Type | Optimal Mixture Ratio | Detection Limit (Minor Contributor) | Key Applications | |
|---|---|---|---|---|
| Standard STRs | Up to 1:10 | ~10-20% | Standard crime scene samples, balanced mixtures | [2] [1] |
| Y-STRs | Up to 1:1000 (female:male) | <1% (male in female background) | Sexual assault evidence with male suspect/female victim | [1] |
| DIP-STRs | Up to 1:1000 | ~0.1% | Extremely unbalanced mixtures, microchimerism, touch DNA | [4] [2] [1] |
| SNP-STRs | Up to 1:40 | ~2.5% | Moderately unbalanced mixtures | [1] |
Experimental Protocols and Validation
DIP Panel Development and Validation
Recent research has established rigorous protocols for developing and validating DIP panels for forensic applications. The following workflow outlines the key stages in establishing a validated DIP panel for forensic use, based on current methodologies [6].
Candidate Marker Selection Criteria
The establishment of a 60-panel DIP system for East Asian populations followed stringent selection criteria [6]:
- Minimum allele frequency (MAF) ≥ 0.1 in target populations
- Biallelic ins/del/delins polymorphisms with allele length variation of 1-20 bp
- Independent inheritance ensured by locating markers on different chromosomes or >5 Mb apart on the same chromosomal arm
- Significant allele frequency differences between populations to enable ancestry inference
- Flanking sequences free of polynucleotides, indels, or other genetic variations
Developmental Validation
Comprehensive validation following SWGDAM guidelines assessed [6]:
- PCR conditions including primer concentration, reaction volume, and thermal cycling parameters
- Sensitivity with serial DNA dilutions to determine the minimum input requirement
- Species specificity using non-human DNA to confirm human specificity
- Stability tests with inhibited and degraded samples
- Mixture analysis to determine performance with mixed DNA profiles
- Reproducibility across multiple operators and instruments
STR Protocol for Population Studies
Standard STR analysis protocols involve [29]:
- DNA extraction using commercial kits or Chelex-100 methods
- Multiplex PCR amplification with commercial kits (e.g., AmpFlSTR Identifiler)
- Capillary electrophoresis on genetic analyzers (e.g., ABI 3500)
- Genotype calling using specialized software (e.g., GeneMapper ID) with comparison to allelic ladders
- Statistical analysis including Hardy-Weinberg equilibrium testing, allele frequency calculation, and forensic parameter estimation using tools like Arlequin and PowerStats
The Scientist's Toolkit
Table 3: Essential Research Reagents and Materials for Forensic Marker Analysis
| Item | Function | Example Applications | |
|---|---|---|---|
| Commercial STR Kits | Multiplex amplification of standard STR loci | Database building, routine casework (e.g., AmpFlSTR Identifiler) | [29] |
| DIP-Specific Primer Panels | Targeted amplification of DIP loci | Ancestry inference, individual identification with DIPs | [6] |
| PCR Master Mix | Enzymatic amplification of target loci | All PCR-based genotyping methods | [6] [30] |
| Capillary Electrophoresis Instrument | Fragment size separation and detection | STR and DIP fragment analysis (e.g., ABI 3500) | [6] [29] |
| Genetic Analyzer Software | Genotype calling and data interpretation | Allele designation for STRs and DIPs (e.g., GeneMapper ID) | [29] [30] |
| Population Genetics Software | Statistical analysis of genotype data | Forensic parameter calculation, HWE testing (e.g., Arlequin, STRAF) | [6] [29] |
| Reference DNA Standards | Quality control and standardization | Ensuring reproducibility across experiments (e.g., 9948) | [6] [30] |
The comparative analysis of DIP panels and STR markers reveals distinct advantages for specific forensic applications. STR markers continue to offer exceptional discrimination power for standard crime scene samples and remain the foundation of national DNA databases worldwide [29] [31].
However, DIP-based panels demonstrate superior performance in specific challenging scenarios:
- Highly unbalanced mixtures, where DIP-STR markers can detect minor contributors at ratios up to 1:1000, significantly outperforming standard STRs [4] [2]
- Degraded DNA samples, where shorter DIP amplicons provide more robust amplification [6] [30]
- Ancestry inference, where the population-specific allele frequencies of DIPs offer valuable biogeographic information [6]
For paternity testing, both marker systems can achieve extremely high combined exclusion probabilities, though STRs typically reach slightly higher CPE values in standard panels [6] [29]. The compound Multi-InDel markers show particularly promising results, with CPD values approaching theoretical perfection and CPE values exceeding 0.999998 in studied populations [30].
The selection between DIP panels and STR markers should be guided by the specific evidentiary context, with STRs remaining optimal for standard applications and DIP-based systems offering powerful solutions for challenging samples that would otherwise yield inconclusive results. Future developments will likely focus on integrated systems that combine the strengths of both marker types to expand forensic capabilities across diverse identification scenarios.
The genetic analysis of unbalanced DNA mixtures, where one individual's DNA dominates a sample, presents a significant challenge across multiple fields, from forensic science to medical diagnostics. In such mixtures, the minor DNA contributor often goes undetected due to the masking effect of the dominant DNA, limiting the utility of conventional genetic profiling methods [1]. This analysis compares the performance of innovative Deletion/Insertion Polymorphism-Short Tandem Repeat (DIP-STR) markers against traditional Short Tandem Repeat (STR) forensic markers within two specialized applications: non-invasive prenatal testing (NIPT) and microchimerism analysis. While STR markers form the current gold standard, their limited sensitivity in detecting minor DNA components below 5-10% of the total mixture has driven the development of more sensitive alternatives like DIP-STR markers [1] [32] [2].
Marker Technology Fundamentals
Short Tandem Repeat (STR) Markers
STR markers analyze regions of DNA containing short, repetitive sequences (typically 2-7 base pairs) that vary considerably in repeat number between individuals. These polymorphisms have become the foundation of forensic identification and relationship testing due to their high discrimination power and ease of amplification using polymerase chain reaction (PCR) followed by capillary electrophoresis analysis [1]. In standard STR analysis, the detection of a minor DNA contributor in a mixed sample requires that contributor to represent at least 10-20% of the total DNA, as PCR amplification bias favors the major component [1] [2]. This limitation poses significant challenges in applications such as prenatal testing, where fetal DNA represents only 3-10% of the cell-free DNA in maternal circulation, and in microchimerism analysis, where foreign DNA may constitute less than 1% of the total [33] [4].
DIP-STR Compound Markers
DIP-STR markers represent an innovative compound approach that pairs a deletion/insertion polymorphism (DIP) with a closely linked STR polymorphism [4]. The strategic design uses allele-specific PCR primers targeting the DIP region (either the insertion "L" allele or deletion "S" allele) to selectively amplify the minor contributor's DNA while suppressing amplification of the major contributor's DNA [1] [9]. The compound nature of these markers generates high-level polymorphism suitable for identity testing, with the linked STR component enhancing discrimination power [4]. This design enables DIP-STR markers to resolve extremely unbalanced two-source DNA mixtures with sensitivity up to 1:1000 minor-to-major DNA ratio, substantially outperforming conventional STR markers [4] [9].
Table 1: Fundamental Characteristics of STR and DIP-STR Markers
| Characteristic | STR Markers | DIP-STR Markers |
|---|---|---|
| Marker Type | Single polymorphism (tandem repeats) | Compound marker (DIP + STR) |
| Detection Sensitivity | 1:10 to 1:20 minor:major DNA ratio | Up to 1:1000 minor:major DNA ratio |
| Amplicon Size | Variable, often >200 bp | Short (average 230 bp) |
| Analysis Method | PCR + capillary electrophoresis | Allele-specific PCR + capillary electrophoresis |
| Polymorphism | High (multiple alleles per locus) | Very high (compound haplotype) |
| Gender Limitations | None (autosomal or Y-chromosome) | None (autosomal markers) |
Application in Non-Invasive Prenatal Testing (NIPT)
Background and Clinical Need
Non-invasive prenatal testing has revolutionized prenatal screening by enabling detection of fetal chromosomal abnormalities through analysis of cell-free fetal DNA (cffDNA) present in maternal blood. cffDNA constitutes approximately 3-10% of the total cell-free DNA in maternal circulation during pregnancy, creating a naturally occurring unbalanced DNA mixture that poses analytical challenges [33] [34]. The primary source of cffDNA is the placenta, with fetal DNA detectable as early as 5-7 weeks of gestation and cleared rapidly from maternal circulation after delivery [33]. Current NIPT methodologies primarily screen for common aneuploidies including trisomy 21 (Down syndrome), trisomy 18 (Edwards syndrome), and trisomy 13 (Patau syndrome) [35] [36].
Performance Comparison of Analytical Approaches
Table 2: Performance Comparison of Prenatal Testing Methods
| Method | Detection Sensitivity | PPV for T21 | PPV for T18 | PPV for T13 | Gestational Age |
|---|---|---|---|---|---|
| STR Analysis | Limited in cffDNA mixtures | Not primary use | Not primary use | Not primary use | Varies |
| DIP-STR Markers | 1:1000 (theoretical) | Research phase | Research phase | Research phase | ≥8 weeks |
| Standard NIPT | 99.25% for T21 [36] | 86.81% [36] | 56.81% [36] | 18.18% [36] | ≥10 weeks |
| Invasive Diagnosis | >99% for major anomalies | >99% | >99% | >99% | 15-20 weeks (amnio) |
The performance of standard NIPT has been demonstrated in large-scale studies. A retrospective analysis of 282,911 pregnant women reported high sensitivity and specificity for common trisomies: sensitivity of 99.25%, 98.33%, and 100.00% for T21, T18, and T13, respectively, with specificities exceeding 99.97% for all three conditions [36]. The positive predictive values (PPV) showed significant variation between trisomies, with T21 demonstrating the highest PPV at 86.81%, followed by T18 at 56.81%, and T13 at 18.18% [36]. These performance metrics are influenced by maternal age, with significantly higher PPVs observed in women over 35 (85.53%) compared to younger women (58.90%) [36].
While DIP-STR markers are not currently used in commercial NIPT, their potential application lies in noninvasive prenatal paternity testing and specific mutation analysis. Research demonstrates that DIP-STRs can target fetal DNA in maternal plasma as early as eight weeks of pregnancy, exploiting the marker's ability to amplify genomic regions from cell-free fetal DNA [1] [9]. This approach can determine paternity by identifying additional DIP-STR alleles inherited from the father in the plasma of pregnant women [1]. In twin pregnancies, DIP-STR analysis can detect two non-maternal DIP-STR alleles in maternal plasma, with the determined alleles differing from maternal alleles [1].
Application in Microchimerism Analysis
Background and Clinical Significance
Microchimerism refers to the presence of a small population of cells or DNA from one individual in another, occurring at levels typically below 1%. This phenomenon occurs naturally during pregnancy (fetal cells in maternal circulation) and following organ transplantation (donor cells in recipient) [4] [34]. In hematopoietic stem cell transplantation (HSCT), monitoring chimerism status is crucial for assessing engraftment, predicting relapse, and guiding immunotherapy interventions [32]. The quantitative follow-up of chimerism proportions has significant clinical utility in transplantation settings, with the reappearance of recipient cells in a patient who had previously achieved complete chimerism potentially indicating impending relapse of the underlying disease [32].
Performance Comparison of Analytical Approaches
Table 3: Performance Comparison in Microchimerism Analysis
| Method | Sensitivity | Quantification Capacity | Informative Loci | Key Applications |
|---|---|---|---|---|
| STR-PCR | 1-5% [32] | Excellent [32] | 3-8 markers needed [37] | Post-HSCT monitoring, engraftment |
| DIP-STR Markers | 0.1-1% (1:1000) [4] | Research phase | Requires population data [1] | DNA microchimerism, forensic applications |
| qPCR (indel) | 0.01-0.1% [32] | Good, but less accurate at high recipient % | 2 markers typically sufficient [32] | Minimal residual disease, early relapse |
STR analysis remains the gold standard technique for chimerism quantification in clinical settings due to its well-proven quantification capacity [32]. The sensitivity of STR-PCR is approximately 1-5%, which can be improved by studying specific leukocyte subsets but may still be insufficient for detecting early signs of complications such as disease relapse [32]. Studies have identified the most informative STR loci for chimerism analysis, with D2S1338, D21S11, D18S51 and FGA representing the most effective markers, allowing direct detection of chimerism in approximately three-quarters of cases [37]. A minimum set of 8 STR markers (D2S1338, D21S11, D18S51, FGA, VWA, D19S433, TH01 and D3S1358) provides at least three informative loci in 95% of cases, enabling effective chimerism monitoring [37].
DIP-STR markers offer significantly enhanced sensitivity for detecting microchimerism, capable of identifying a minor DNA component contributing only 0.1% to the mixed sample [4] [9]. This sensitivity advantage makes DIP-STRs particularly valuable for applications involving extremely low levels of foreign DNA, such as in fetomaternal microchimerism long after pregnancy or in solid organ transplantation monitoring [4]. Unlike Y-STR markers that require gender mismatch, DIP-STR markers can be applied regardless of the sex of the contributors, significantly expanding their potential applications [1] [2].
Experimental Protocols and Methodologies
DIP-STR Analysis Workflow
The DIP-STR methodology begins with DNA extraction from the mixed biological sample, followed by careful primer design. The primer design phase involves creating allele-specific primers targeting the DIP region: one primer set for the deletion (S allele) and another for the insertion (L allele) [4] [9]. These primers are strategically designed to exploit the DIP variation to selectively amplify the minor contributor's DNA. The critical allele-specific PCR amplification step follows, employing reaction conditions that favor the amplification of the minor DNA component while suppressing amplification of the major DNA [1] [4]. Successful amplification requires the minor and major donors to have opposite DIP genotypes (e.g., SS/LL or LL/SS), creating what are termed "informative genotypes" [1]. The resulting PCR products then undergo capillary electrophoresis for fragment analysis, similar to conventional STR typing [1]. The final genotyping step identifies the STR alleles linked to the targeted DIP allele, generating a haplotype profile of the minor DNA contributor [9]. This is followed by comprehensive profile interpretation and statistical analysis to determine the evidentiary value of the match [2].
STR-Based Chimerism Analysis Protocol
Standard STR-based chimerism analysis begins with sample collection from peripheral blood or bone marrow, followed by DNA extraction and quantification [32] [37]. The next step involves multiplex STR PCR amplification using carefully selected marker panels. Research indicates that a minimum set of 8 optimally informative STR markers (D2S1338, D21S11, D18S51, FGA, VWA, D19S433, TH01, and D3S1358) provides at least three informative loci in 95% of cases [37]. The PCR products are then separated by capillary electrophoresis with fluorescence detection, allowing identification of alleles from different contributors based on fragment size [32]. Sophisticated peak analysis follows, examining both peak height and area to distinguish between donor and recipient alleles [32] [37]. The final chimerism quantification calculates the relative proportions of donor and recipient DNA based on allele ratios at informative loci, with results categorized as complete chimerism (only donor DNA), mixed chimerism (both donor and recipient DNA detectable), or increasing mixed chimerism (rising recipient DNA percentage potentially indicating relapse) [32].
The Scientist's Toolkit: Essential Research Reagents
Table 4: Essential Research Reagents for DIP-STR and STR Analysis
| Reagent/Kit | Function | Application Context |
|---|---|---|
| AmpFℓSTR Identifiler Plus | Multiplex STR amplification | Forensic identification, chimerism analysis |
| Mentype Chimera | Commercial STR chimerism kit | Post-transplant monitoring |
| DIP-STR Allele-Specific Primers | Custom primers for minor DNA amplification | Unbalanced mixture deconvolution |
| Proteinase K | Cell lysis and DNA release | DNA extraction from biological samples |
| Maxwell Blood DNA Purification Kit | Automated nucleic acid extraction | High-quality DNA purification |
| Affymetrix CytoScan 750K Array | Chromosomal microarray analysis | CNV detection in prenatal diagnosis |
| BGISEQ-500 Platform | Massively parallel sequencing | NIPT for aneuploidy screening |
The comparative analysis of DIP-STR and STR markers reveals distinct advantage profiles for these technologies in niche applications involving unbalanced DNA mixtures. STR markers maintain their position as the gold standard for applications requiring robust quantification and where the minor DNA component exceeds 5% of the total mixture, particularly in chimerism monitoring after hematopoietic stem cell transplantation [32] [37]. Their advantages include well-established population databases, standardized interpretation guidelines, and excellent quantitative capabilities at moderate sensitivity levels.
DIP-STR markers demonstrate superior sensitivity for detecting extremely minor DNA components (as low as 0.1%) in unbalanced mixtures, offering significant potential for noninvasive prenatal paternity testing, forensic analysis of mixed stains, and detection of microchimerism at very low levels [1] [4] [9]. Their key advantages include applicability regardless of contributor genders, higher polymorphism through compound markers, and ability to work with degraded DNA due to shorter amplicon sizes.
Future development efforts are focusing on expanding DIP-STR marker panels, with recent research identifying approximately 3000 candidate DIP-STRs from whole-genome sequencing data and validating 23 novel markers to create a more comprehensive analytical panel [9]. The development of multiplex DIP-STR assays using either capillary electrophoresis or massively parallel sequencing platforms represents the next frontier, which would reduce DNA consumption, increase efficiency, and provide sufficient informative markers for comprehensive minor contributor characterization across various applications [9].
Overcoming Analytical Hurdles in Complex Forensic Samples
Addressing PCR Amplification Bias in Unbalanced Mixtures
In forensic science and clinical diagnostics, samples containing DNA from multiple individuals in highly unbalanced ratios present a significant analytical challenge. This imbalance induces PCR amplification bias, where the major contributor's DNA amplifies more efficiently, masking the minor contributor's genetic profile. Conventional Short Tandem Repeat (STR) markers often fail when the minor DNA component falls below 10-20% of the total DNA due to competitive priming and stochastic effects during Polymerase Chain Reaction (PCR) [4] [1]. This comparative analysis evaluates the performance of innovative genetic markers against traditional forensic methods, focusing on their efficacy in resolving severely unbalanced DNA mixtures.
The Fundamental Challenge: PCR Amplification Bias
PCR amplification bias describes the non-random, preferential amplification of certain DNA sequences over others during the PCR process. In the context of unbalanced mixtures, this bias is exponentially amplified, causing the dominant DNA profile to overwhelm that of the minor contributor [38]. Several factors contribute to this effect:
- Template Sequence Composition: GC-rich regions or sequences with homopolymers can affect polymerase efficiency [38].
- Primer Mismatches: Even a single mismatch between the primer and template DNA can suppress amplification efficiency by an order of magnitude or more [38].
- Stochastic Effects: In early PCR cycles, low-quantity templates may by chance not be amplified, leading to significant drift in final product ratios [39].
- PCR Selection: Certain templates are inherently favored due to properties of their gene sequences or flanking regions, leading to reproducible over-amplification [39].
The following diagram illustrates how amplification bias occurs in a mixed DNA sample, leading to the loss of the minor contributor's profile.
Comparative Analysis of Methodologies
Conventional Autosomal STR Markers
Traditional forensic STR profiling, while the gold standard for single-source samples, faces considerable limitations with unbalanced mixtures.
- Performance Limitation: Standard STR analysis requires the minor DNA component to represent at least 10-20% of the total DNA for reliable detection, and even more (≥20%) for unambiguous identification of all minor alleles [4] [1].
- Root Cause: During PCR, primers compete for binding, and the abundant major DNA outcompetes the scarce minor DNA, leading to its effective disappearance from the final profile [1].
Y-Chromosome STRs (Y-STRs)
Y-STRs target the male-specific Y chromosome and are useful in specific scenarios like sexual assault cases involving female victim and male perpetrator DNA.
- Advantage: Effective for detecting male DNA in a high female DNA background [1].
- Disadvantages:
Deletion/Insertion Polymorphism-STR (DIP-STR)
This compound marker combines a biallelic Deletion/Insertion Polymorphism (DIP) with a nearby STR.
- Principle: Allele-specific primers are designed to target either the insertion ("L") or deletion ("S") allele of the DIP. This allows selective amplification of the minor contributor's DNA if it possesses a unique DIP allele not present in the major contributor [4] [1].
- Performance: Demonstrates superior sensitivity, capable of detecting a minor DNA contributor at ratios as low as 1:1,000 [4] [1]. The linked STR then provides high discrimination power for individual identification.
Allele-Specific INDEL Real-Time PCR
This method uses real-time PCR with separate, primer-specific amplifications for each allele of an Insertion/Deletion (INDEL) polymorphism.
- Principle: Two separate real-time PCR reactions are run with primers specific to each INDEL allele. The difference in cycle threshold (Ct) values between the two reactions (D-value) indicates whether a sample is from a single source or a mixture [40].
- Performance: Reported detection limits for the minor DNA component range from 1:50 to 1:1000, depending on the specific INDEL marker used [40].
Single Nucleotide Polymorphism-STR (SNP-STR)
A compound marker linking a biallelic Single Nucleotide Polymorphism (SNP) with a nearby STR.
- Principle: Similar to DIP-STR, it uses allele-specific primers (ARMS-PCR) for the SNP to target a genomic region unique to the minor DNA, reducing the masking effect. The STR provides polymorphism [1].
- Performance: Less sensitive than DIP-STR, with a reported detection limit for the minor component of approximately 1:40 [1].
Table 1: Quantitative Performance Comparison of DNA Mixture Analysis Methods
| Method | Detection Limit (Minor:Major) | Key Advantage | Primary Limitation |
|---|---|---|---|
| Conventional STR | 1:5 to 1:10 [4] [1] | Gold standard, high discrimination for balanced mixtures | Fails with highly unbalanced mixtures |
| Y-STR | Varies by context | Effective for male DNA in female background | Sex-specific; identifies lineage, not individual [1] |
| DIP-STR | 1:1,000 [4] [1] | Extremely high sensitivity; autosomal | Requires informative DIP alleles [1] |
| Allele-Specific INDEL (qPCR) | 1:50 to 1:1,000 [40] | Quantitative (Ct values); high sensitivity | Requires separate reactions and D-value analysis [40] |
| SNP-STR | ~1:40 [1] | Automosomal; compound marker | Lower sensitivity than DIP-STR [1] |
Table 2: Qualitative Feature Comparison of DNA Mixture Analysis Methods
| Method | Multiplexing Potential | Required Analysis Platform | Ideal Application |
|---|---|---|---|
| Conventional STR | High (commercial kits) | Capillary Electrophoresis | Single-source or balanced mixture evidence |
| Y-STR | High (commercial kits) | Capillary Electrophoresis | Sexual assault cases (male-female mixtures) |
| DIP-STR | Moderate (panel development) | Capillary Electrophoresis | Extreme unbalanced mixtures (forensic & microchimerism) [4] |
| Allele-Specific INDEL (qPCR) | Low (singleplex or fewplex) | Real-Time PCR | Mixture detection and quantification [40] |
| SNP-STR | Moderate (panel development) | Capillary Electrophoresis | Unbalanced mixtures where DIP-STR is not informative |
Experimental Protocols and Data
DIP-STR Protocol and Performance Data
Workflow Overview:
- Marker Selection: Identify DIPs located within 500 base pairs of a polymorphic STR [4].
- Primer Design: Create two sets of forward primers: one allele-specific for the "long" (L) insertion and another for the "short" (S) deletion. A common reverse primer targets the linked STR [4] [1].
- PCR Amplification: Perform separate amplifications with the L and S-specific primers.
- Capillary Electrophoresis: Analyze PCR products to separate and detect the STR alleles linked to the specifically amplified DIP allele [1].
Table 3: Exemplary DIP-STR Markers and Their Performance [4]
| DIP-STR Marker | Chromosome | DIP Type | STR Repeat | Performance in Mixtures |
|---|---|---|---|---|
| MID1013–D5S490 | 5q23.2 | -/CCAG | GT | Effective in simulated mixtures |
| MID1950–D20S473 | 20p13 | -/ATT | TTA | Effective in simulated mixtures |
| rs11277790–D10S530 | 10q25.1 | -/TCCAACT | GT | Effective in simulated mixtures |
Allele-Specific INDEL Real-Time PCR Protocol
Workflow Overview:
- INDEL Selection: Choose polymorphic INDELs with high Minor Allele Frequency (MAF >0.2) [40].
- Separate Amplification: Perform two parallel real-time PCR reactions with primer sets specific to each INDEL allele.
- Ct Value Analysis: Calculate the difference in Ct values (D-value) between the two reactions.
- Interpretation: A large D-value indicates a homozygous sample (LL or SS), while a D-value between 0.86 and 5.11 (for the studied 14 INDELs) indicates a mixture [40].
- Confirmation: Final genotypes can be confirmed by electrophoresis [40].
Table 4: Performance of Allele-Specific INDEL Method in Chinese Han Population [40]
| INDEL Marker Example | Template DNA Range | Average D-Value (Single Source) | Minor DNA Detection Limit |
|---|---|---|---|
| rs397782455 | 0.3125 ng - 1.25 ng | LS: 0.31 ± 0.14; LL: 6.96 ± 1.05; SS: 7.20 ± 1.09 | 1:50 |
| rs397832665 | 0.3125 ng - 1.25 ng | LS: 0.31 ± 0.14; LL: 6.96 ± 1.05; SS: 7.20 ± 1.09 | 1:100 |
| 9 other INDELs | 0.3125 ng - 1.25 ng | LS: 0.31 ± 0.14; LL: 6.96 ± 1.05; SS: 7.20 ± 1.09 | 1:500 to 1:1000 |
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 5: Key Reagents and Materials for Advanced DNA Mixture Analysis
| Item | Function/Description | Example Application |
|---|---|---|
| Allele-Specific Primers | Primers designed to perfectly match one allele of a polymorphism (DIP/SNP) and mismatch others, enabling selective amplification. | Core component of DIP-STR and SNP-STR protocols [4] [1]. |
| High-Fidelity DNA Polymerase | PCR enzymes with proofreading activity to reduce errors during amplification, crucial for accurate sequencing and complex assay development. | Optimized library amplification for sequencing; general high-fidelity PCR [41]. |
| Betaine (PCR Additive) | A chemical additive that reduces the melting temperature of DNA, helping to amplify GC-rich templates more uniformly and reduce base-composition bias. | Improving amplification evenness in libraries with wide %GC ranges [41]. |
| Real-Time PCR Instrument | Equipment that monitors PCR amplification in real-time using fluorescence, allowing for quantification and Ct value determination. | Essential for allele-specific INDEL Ct value and D-value analysis [40]. |
| Capillary Electrophoresis (CE) System | Instrumentation for separating DNA fragments by size and detecting fluorescently labeled primers, the standard for STR fragment analysis. | Standard platform for analyzing STR, DIP-STR, and SNP-STR amplicons [1] [7]. |
| Degraded DNA Analysis Kit | Commercial kits optimized for shorter amplicon sizes to improve success rates with degraded or challenging forensic samples. | Profiling from degraded or formalin-fixed samples [7]. |
The analysis of unbalanced DNA mixtures requires moving beyond conventional STR typing to overcome inherent PCR amplification biases. While Y-STRs serve a specific niche, the emerging compound marker systems—DIP-STR and SNP-STR—offer significantly enhanced capabilities for autosomal analysis regardless of contributor sex.
DIP-STR markers currently represent the most sensitive option, capable of detecting a minor DNA component at a 1:1,000 ratio, a 100-fold improvement over traditional STRs [4] [1]. The allele-specific INDEL real-time PCR method provides a powerful alternative with the added benefit of quantification through Ct value analysis, achieving similar high sensitivity [40]. The choice between these methods depends on specific application needs, available instrumentation, and the requirement for absolute sensitivity versus quantitative data. These advanced techniques are poised to revolutionize the analysis of challenging forensic evidence and clinical samples such as circulating fetal DNA in maternal plasma or donor DNA in transplant recipients.
Strategies for Analyzing Degraded and Low-Quantity DNA Evidence
The analysis of degraded and low-quantity DNA evidence remains one of the most significant challenges in forensic science. Traditional methods often fail to produce usable profiles from compromised samples, potentially leaving critical criminal cases unsolved. This comparative analysis examines the performance of Deletion/Insertion Polymorphism (DIP) panels against conventional Short Tandem Repeat (STR) markers for processing challenging forensic evidence. Emerging technologies and marker systems are reshaping forensic workflows, enabling scientists to recover genetic information from samples once considered unusable. Understanding the relative strengths, limitations, and optimal applications of these systems is essential for advancing forensic capabilities and delivering justice in complex cases.
Technical Comparison: DIP Panels vs. STR Markers
The fundamental differences between DIP and STR markers directly impact their performance with degraded and low-quantity DNA evidence. The table below summarizes the key technical characteristics of each system:
Table 1: Technical Comparison of DIP Panels and STR Markers
| Characteristic | DIP Panels | STR Markers |
|---|---|---|
| Marker Type | Insertion/Deletion length polymorphisms | Tandem repeat number variations |
| Mutation Rate | ~10⁻⁸ (Lower) [6] | ~10⁻³ (Higher) [14] |
| Stutter Peaks | None [6] [16] | Present, can complicate interpretation [14] |
| Amplicon Size | Typically <200 bp [6] [16] | Often >200 bp, sometimes significantly larger [14] |
| Analysis Platform | Capillary electrophoresis or NGS [6] | Primarily capillary electrophoresis [14] |
| Polymorphism Nature | Biallelic [6] | Multiallelic [14] |
| Ancestral Information | Rich biogeographic data [6] [16] | Limited ancestry inference capability |
DIP markers exhibit significantly lower mutation rates compared to STRs, enhancing their stability across generations and making them particularly valuable for kinship analysis [6] [14]. The absence of stutter peaks in DIP analysis eliminates a major interpretation challenge present in STR profiling, thereby increasing typing accuracy, especially in mixed samples [6] [16].
The most critical advantage for degraded DNA analysis is the shorter amplicon size of DIP markers. DNA degradation fragments molecules into smaller pieces, making shorter targets more likely to amplify successfully. DIP panels are specifically designed with amplicons limited to 200bp or less to maximize recovery from compromised samples [6] [16]. In contrast, traditional STR systems often struggle with degraded DNA because their longer amplicon sizes exceed the fragment length of damaged DNA [14].
Performance Metrics and Experimental Data
Forensic Efficiency Parameters
Recent validation studies have quantified the performance of DIP panels for forensic applications. The table below summarizes key efficiency parameters reported for various DIP systems:
Table 2: Forensic Efficiency Metrics of DIP Panels
| Panel Size | Population Studied | Combined Probability of Discrimination | Cumulative Probability of Exclusion | Reference |
|---|---|---|---|---|
| 60-plex DIP panel | East Asian | 0.999999999999 | 0.9937 | [6] |
| 43 A-DIP panel | Chinese Yi | 1.11433E-18 (CMP*) | 0.999610217 | [16] |
| 43 A-DIP panel | Chinese Hani | 8.24299E-19 (CMP*) | 0.999629285 | [16] |
| 43 A-DIP panel | Chinese Miao | 4.21721E-18 (CMP*) | 0.999582084 | [16] |
*CMP: Cumulative Match Probability
The 60-plex DIP panel demonstrated exceptional discrimination power with a combined probability of discrimination exceeding 0.999999999999, indicating near-certain individualization capability [6]. Similarly, the 43 A-DIP panel showed remarkable performance across multiple populations, with cumulative match probabilities reaching as low as 4.21721E-18 in the Miao population [16]. These values meet or exceed forensic standards for individual identification and kinship analysis.
Performance with Degraded DNA
Experimental validation of the 60-plex DIP panel demonstrated robust performance with poor-quality samples, yielding reliable genotypes even from degraded DNA [6]. The panel's design specifically prioritized shorter amplicons to accommodate the fragmentary nature of degraded evidence, with validation studies confirming this advantage. This capability is particularly valuable for challenging forensic cases involving ancient remains, disaster victims, or evidence exposed to environmental stressors [42].
In low-template DNA mixtures (50-100 pg per contributor), STR typing exhibits significant stochastic effects including allelic drop-out, drop-in, and peak height imbalance [43]. These phenomena complicate interpretation and reduce reliability. DIP markers' lower mutation rates and absence of stutter peaks provide inherent advantages in such challenging scenarios.
Analysis of Mixed Samples
DIP-STR markers, a compound marker system, show particular promise for analyzing extremely unbalanced mixtures where the minor contributor represents less than 10% of the total DNA [2]. In such cases, standard STR analysis often fails to detect the minor contributor's profile because it is masked by the major contributor. DIP-STR markers can successfully characterize the minor contributor in these situations, making them particularly valuable for sexual assault cases where the victim's DNA vastly outweighs the perpetrator's [2].
Figure 1: Analytical Workflow for Unbalanced DNA Mixtures
Experimental Protocols and Methodologies
DIP Panel Validation Protocol
The developmental validation of the 60-plex DIP panel followed rigorous scientific guidelines recommended by the Scientific Working Group on DNA Analysis Methods (SWGDAM) [6]. The validation encompassed multiple experimental parameters:
- PCR Condition Optimization: Testing of reaction mix volumes (0.5× to 1.5×), primer concentrations, and reaction volumes (5-25 μL)
- Thermal Cycling Parameters: Denaturation temperature gradients (89-99°C), annealing temperature optimization (55-65°C), and cycle number testing (21-27 cycles)
- Sensitivity Studies: Serial dilution experiments to determine the minimum input DNA requirement
- Species Specificity: Assessment of cross-reactivity with non-human DNA
- Stability Studies: Performance evaluation with degraded and inhibited samples
- Mixture Analysis: Determination of mixture detection thresholds and interpretation guidelines
- Reproducibility Testing: Inter-laboratory and intra-laboratory replication studies
- Case-Type Samples: Validation with simulated case samples including blood, saliva, and touch DNA
This comprehensive validation protocol ensures that DIP panels perform reliably across the diverse range of evidentiary materials encountered in forensic casework [6].
STR Kit Comparative Analysis Methodology
Comparative studies of STR kits employ standardized methodologies to assess performance with challenging samples:
- Serial Dilution Experiments: DNA samples diluted to concentrations ranging from 3 pg to 420 pg and amplified in triplicate using different PCR cycle numbers (28, 29, 30 cycles) [26]
- Sensitivity Analysis: Determination of allelic drop-out probabilities at various template concentrations
- Stutter Percentage Calculation: Quantification of stutter product formation for each locus
- Heterozygote Balance Assessment: Measurement of peak height ratios for heterozygous alleles
- Inter-locus Balance: Evaluation of peak height consistency across different loci in the multiplex
- Degraded DNA Simulation: Artificial degradation of samples through heat and humidity exposure [43]
These methodologies enable objective comparison of STR kit performance with low-template and degraded DNA, informing kit selection for specific casework scenarios [43] [26].
Emerging Technologies and Future Directions
Next-Generation Sequencing (NGS)
Next-Generation Sequencing represents a paradigm shift in forensic DNA analysis, enabling simultaneous examination of hundreds to thousands of genetic markers from a single sample [44] [45]. Unlike capillary electrophoresis, which analyzes approximately 24 markers, NGS can interrogate over 150 genetic markers, providing significantly more data from limited evidence [44]. This technology is particularly valuable for degraded samples because it can sequence shorter DNA fragments that would be uninformative with traditional methods.
NGS facilitates the analysis of dense single nucleotide polymorphisms (SNPs) that provide investigative leads through forensic genetic genealogy, biogeographical ancestry inference, and phenotypic prediction [45]. This capability has proven revolutionary for cold cases where standard STR typing has failed to produce actionable leads.
Alternative Marker Systems
Beyond DIP panels, several specialized marker systems show promise for challenging evidence:
- Mini-STRs: These systems reduce flanking regions around STR repeats, producing shorter amplicons (70-150 bp) that are more likely to amplify successfully from degraded DNA [14]
- SNP-STR Markers: Compound markers that combine single nucleotide polymorphisms with STRs to improve mixture deconvolution [2]
- Y-STRs: Useful specifically for male-minor mixtures in female-major backgrounds, though limited by gender dependency [2]
Advanced DNA Extraction and Preservation
Emerging technologies in DNA extraction and preservation significantly impact the analysis of degraded evidence:
- Miniaturized Portable DNA Extraction Kits: Enable rapid on-site processing, reducing sample degradation during transport [46]
- Automated Extraction Systems: Improve consistency and reduce contamination risk (e.g., PrepFiler Express with Automate Express platform) [46]
- Advanced Preservation Techniques: Specialized storage materials with desiccants and stabilizing agents prevent DNA degradation in field conditions [46]
The Scientist's Toolkit: Essential Research Reagents
Table 3: Essential Research Reagents for Degraded DNA Analysis
| Reagent/Kit | Function | Application Context |
|---|---|---|
| AGCU DIP 60 Kit | Multiplex amplification of 56 A-DIPs, 3 Y-DIPs, and Amelogenin | Individual identification and ancestry inference [6] |
| Investigator DIPplex Kit | First commercial DIPplex panel for forensic identification | Standardized DIP analysis [16] |
| PowerPlex ESI17 System | STR multiplex amplifying extended ESS loci | Comparative degraded DNA studies [43] [26] |
| AmpFℓSTR NGMSElect | STR multiplex for crime scene samples | Kit performance comparisons [26] |
| PrepFiler Express Kit | Automated DNA extraction | Rapid processing of challenging samples [46] |
| Organic Extraction Reagents (Phenol-Chloroform) | DNA purification from difficult substrates | Removal of PCR inhibitors [42] |
| Quantifiler Trio Kit | DNA quantification and degradation assessment | Quality assessment prior to amplification [42] |
The comparative analysis of DIP panels versus STR markers reveals a nuanced landscape for degraded and low-quantity DNA evidence analysis. DIP panels offer significant advantages for severely degraded samples due to their shorter amplicon sizes, absence of stutter peaks, and lower mutation rates. STR systems, particularly newer multiplexes with mini-STR loci, remain highly effective for moderately compromised evidence and benefit from extensive database infrastructure.
The optimal strategy involves contextual selection of analytical approaches based on sample quality, quantity, and case objectives. For extremely challenging samples—including highly degraded remains, unbalanced mixtures, and cold cases where previous methods have failed—DIP panels and emerging NGS technologies provide powerful alternatives. As forensic science continues to evolve, the integration of multiple marker systems and advanced technologies will expand our capability to deliver justice from even the most compromised biological evidence.
Mitigating Stutter Artifacts and Allelic Dropout
The analysis of complex forensic DNA evidence, particularly mixtures where a minor contributor is masked by a major donor, presents significant challenges for conventional genetic markers. Stutter artifacts and allelic dropout are two principal limitations that can obscure results and complicate interpretation. Stutter peaks, which are typically one repeat unit smaller than the true allele, arise from slipped-strand mispairing during PCR amplification and can be mistaken for true alleles in mixed samples [47]. Allelic dropout occurs when stochastic effects prevent the amplification of one or more alleles, particularly in low-template or highly unbalanced DNA mixtures [48]. This comparative analysis examines how DIP panels (Deletion/Insertion Polymorphisms) and traditional STR markers (Short Tandem Repeats) address these challenges, providing forensic researchers with data-driven insights for method selection.
Molecular Mechanisms and Technical Foundations
STR Markers and Their Limitations
Standard STR profiling relies on amplifying polymorphic microsatellite regions with core repeat units of 2-6 base pairs. The stutter phenomenon in STR analysis is well-characterized, with stutter peaks typically ranging from 5% to 15% of the main allele peak height depending on repeat structure [47]. Several factors influence stutter rates: dinucleotide repeats exhibit higher stutter percentages than tri- or tetranucleotide repeats; more homogeneous repeats produce higher stutter; and longer alleles within a locus generally show increased stutter [47]. These artifacts become particularly problematic in unbalanced mixtures, where stutter peaks from major donor alleles can mimic true alleles from minor contributors, complicating deconvolution.
DIP-STR Compound Markers: A Novel Approach
DIP-STR markers represent an innovative class of compound genetic markers that pair a biallelic deletion/insertion polymorphism (DIP) with a closely linked short tandem repeat (STR) [4] [48]. The key innovation lies in the primer design: two allele-specific primers target either the insertion (L - long allele) or deletion (S - short allele) variant of the DIP, while a third primer targets the adjacent STR region [48]. This design enables allele-specific amplification that preferentially targets minor DNA components in mixtures when they possess DIP alleles not shared with the major contributor [4].
The DIP-STR mechanism significantly reduces stutter artifacts because the allele-specific amplification minimizes PCR slippage at the STR region. Furthermore, the compound nature generates highly polymorphic haplotypes suitable for identity testing [48]. This approach maintains the advantages of standard STR profiling while adding specificity for mixture deconvolution.
Figure 1: DIP-STR Analysis Workflow. The process begins with assessing DIP genotypes to identify cases where the minor contributor has unique DIP alleles not shared with the major contributor, enabling selective amplification.
Comparative Performance Analysis
Quantitative Comparison of Key Parameters
Table 1: Direct comparison of STR and DIP-STR markers across critical forensic parameters
| Parameter | STR Markers | DIP-STR Markers | Experimental Basis |
|---|---|---|---|
| Stutter Artifacts | 5-15% of main allele (higher for dinucleotides) [47] | Significantly reduced or undetectable [1] | CE analysis of amplified products |
| Minor Contributor Detection | 1:10 to 1:20 ratio [4] | Up to 1:1000 ratio [48] | Serial dilution experiments with control DNA |
| Typical Amplicon Size | Varies (often >200 bp) | 146-271 bp (forensic-optimized set) [48] | Fragment analysis on CE systems |
| Mutation Rate | ~10⁻³ per locus per generation | ~10⁻⁸ for DIP component [6] | Population pedigree studies |
| Mixture Resolution | Limited in highly unbalanced mixtures | Effective for two-person unbalanced mixtures [24] | Analysis of simulated mixed samples |
| Gender Independence | Yes | Yes | Testing across different sex combinations |
Experimental Evidence in Forensic Applications
Sensitivity in Unbalanced Mixtures
Comprehensive validation studies demonstrate the superior sensitivity of DIP-STR markers in resolving extremely unbalanced mixtures. In controlled experiments, DIP-STRs successfully genotyped minor contributors present at ratios as low as 1:1000 (0.1%) when using optimized marker sets [48]. This performance substantially exceeds the capabilities of standard STR profiling, which typically requires the minor component to represent at least 5-10% of the total DNA [4] [24]. The detection threshold remains reliable with template DNA quantities as low as 0.03-0.1 ng for the minor component, making DIP-STRs particularly suitable for touch DNA evidence [48].
Performance on Challenging Touch DNA Evidence
A direct comparative study on simulated touch DNA samples highlights the practical advantages of DIP-STR markers. Researchers analyzed 71 unbalanced two-source contact traces created using crime-associated substrates [24]. The results demonstrated that DIP-STRs detected minor contributors of any sex in 54 out of 71 traces (76%), while Y-STRs (limited to male minor/female major mixtures) detected the minor male in only 14 out of 71 traces [24]. This confirms the broader applicability of DIP-STR markers for general forensic casework regardless of contributor gender combinations.
Table 2: Marker performance comparison on touch DNA samples [24]
| Marker Type | Traces with Detected Minor DNA | Gender Limitations | Informative Markers per Sample |
|---|---|---|---|
| Y-STR | 14/71 traces | Male minor contributor only | 13-23 loci typically used |
| DIP-STR | 54/71 traces | No limitations | 1-4 informative markers detected |
| Standard STR | <18 traces with partial profiles | No limitations | ≤5 loci with minor alleles |
Enhanced Specificity and Reduced Artifacts
The allele-specific priming mechanism of DIP-STR markers substantially reduces PCR artifacts that complicate STR analysis. Unlike standard STR profiling that produces characteristic stutter peaks, DIP-STR markers demonstrate minimal to no stutter artifacts due to the targeted amplification approach [1]. This improvement is particularly valuable for mixture interpretation, where stutter peaks from major contributors often obscure true alleles from minor donors. Additionally, the selection of DIP-STR markers with tri-, tetra-, and pentanucleotide repeats in forensic-optimized panels further minimizes stutter potential compared to dinucleotide repeats common in early STR systems [48].
Experimental Protocols and Methodologies
DIP-STR Validation Protocol
The experimental validation of DIP-STR markers follows rigorous forensic standards to establish reliability and sensitivity:
Marker Selection Criteria: Optimal DIP-STR candidates are identified based on several criteria: DIP and STR polymorphisms located within 200 bp, DIP minor allele frequency >0.2 in relevant populations, exclusion of dinucleotide repeats, and physical separation on different chromosomes or chromosomal arms to ensure independence [48]. Recent studies have leveraged whole-genome sequencing data from sources like the Genome Aggregation Database (gnomAD) to identify hundreds of potential DIP-STR candidates throughout the genome [9].
Mixture Simulation Experiments: Validation studies prepare serial dilutions of control DNA samples to create simulated mixtures with precisely defined ratios (1:10, 1:100, 1:1000). These are amplified using allele-specific DIP-STR primers under optimized cycling conditions [48]. The standard PCR protocol includes an initial denaturation at 94°C, followed by 25-30 cycles of denaturation (90°C), allele-specific annealing (60°C), and extension (72°C) [6].
Analysis and Interpretation: Amplification products are separated by capillary electrophoresis and analyzed using standard fragment analysis software. A successful result is recorded when the minor contributor's alleles are detected above the analytical threshold with correct heterozygous balance [24]. The threshold for reliable detection is typically established at 1:1000 minor:major DNA ratio for the most sensitive markers [48].
Protocol for Touch DNA Analysis
The application of DIP-STR markers to touch DNA evidence requires specific methodological considerations:
Sample Collection and DNA Extraction: Touch DNA samples are collected from relevant substrates using standard forensic swabbing techniques. DNA extraction follows established protocols, with potential modifications for low-yield samples [24]. The Chelex-100 extraction method has been successfully employed in validation studies [30].
Informative Marker Selection: Prior knowledge of contributor genotypes enables selection of the most informative DIP-STR markers, though multiplex panels can be applied without prior genotyping. The probability of informative genotypes depends on population allele frequencies and can be calculated for specific population groups [48].
Amplification and Detection: Given the typically low DNA yield from touch evidence, amplification conditions may be optimized with increased cycle numbers (up to 30 cycles) while maintaining strict contamination controls [24]. Multi-capillary instruments allow simultaneous analysis of multiple DIP-STR markers labeled with different fluorescent dyes.
Research Reagent Solutions
Table 3: Essential research reagents and materials for DIP-STR analysis
| Reagent/Material | Function | Specification Notes |
|---|---|---|
| Allele-Specific Primers | Target DIP alleles unique to minor contributor | Designed with 3' end matching DIP variant; possible deliberate mismatch at second-to-last base to enhance specificity [1] |
| STR Primer | Amplify linked STR region | Positioned opposite DIP primer to generate amplicon encompassing both polymorphisms |
| PCR Master Mix | Amplification reaction | Optimized magnesium concentration (typically 1.5-2.0 mM) and inclusion of BSA for inhibitor resistance [6] |
| DNA Quantitation Kit | Measure template DNA concentration | Fluorometric methods preferred for accuracy with low-yield samples |
| Capillary Electrophoresis System | Fragment separation and detection | Standard forensic genetics platforms (e.g., ABI 3500 series) with appropriate size standards [30] |
| Positive Control DNA | Validation of amplification | Characterized reference standards with known DIP-STR genotypes (e.g., 9948) [6] |
Discussion and Future Perspectives
The comparative analysis demonstrates that DIP-STR markers offer significant advantages for resolving unbalanced DNA mixtures by effectively mitigating stutter artifacts and allelic dropout. The capability to detect minor contributors at 1:1000 ratios represents a 100-fold improvement over standard STR methods [4] [48]. This enhanced sensitivity, combined with minimal stutter artifacts, positions DIP-STR technology as a powerful solution for challenging forensic evidence including touch DNA.
Future development efforts are focusing on expanding multiplex DIP-STR panels to increase the probability of informative markers without requiring prior genotype knowledge. Recent research has identified hundreds of potential DIP-STR candidates through analysis of whole-genome sequencing data, with empirical validation of 30 forensic-approved markers [9]. Additionally, compatibility with massively parallel sequencing platforms may further enhance mixture deconvolution capabilities while maintaining the stutter-reduction benefits of the DIP-STR approach [9].
For forensic researchers and drug development professionals working with complex biological mixtures, DIP-STR markers provide a robust, sensitive, and specific alternative to conventional STR profiling. The methodology is particularly valuable for cases involving touch DNA, prenatal testing using cell-free fetal DNA, transplantation monitoring, and other applications where target DNA is present in trace amounts against a complex background [4] [1]. As validation studies continue across diverse populations and implementation guidelines are refined, DIP-STR technology promises to expand the boundaries of recoverable genetic information from challenging forensic and clinical samples.
Bioinformatic Pipelines for Data Analysis and Genotype Calling
This guide provides a comparative analysis of the bioinformatic pipelines used for Deletion/Insertion Polymorphism (DIP) panels versus traditional Short Tandem Repeat (STR) markers in forensic genetics. The focus is on their application in resolving a key forensic challenge: the analysis of unbalanced DNA mixtures.
A primary challenge in forensic DNA analysis is the interpretation of mixtures where two or more individuals have contributed DNA to a sample. In unbalanced mixtures, the DNA of a minor contributor (e.g., a suspect) can be masked by a large excess (e.g., 10- to 1000-fold) of DNA from a major contributor (e.g., a victim). Conventional autosomal STRs, the gold standard for human identification, often fail to detect the minor contributor when their DNA represents less than 5-10% of the total mixture due to PCR amplification bias and the masking effect of the major DNA profile [1] [2].
To address this limitation, alternative marker systems have been developed. Y-chromosome STRs (Y-STRs) are effective for detecting male DNA in a high female DNA background but are useless for same-sex mixtures or for detecting female minor contributors [1] [24]. The DIP-STR marker is a compound marker designed specifically to target a minor contributor's DNA, irrespective of the donors' sexes [4].
Marker Technology and Principle of Analysis
The fundamental difference between STR and DIP-STR markers dictates the subsequent bioinformatic analysis required for genotype calling.
Short Tandem Repeats (STRs)
- Principle: STRs are regions of DNA with a short, repetitive sequence (e.g., AGAT) that is repeated a variable number of times. This length polymorphism is the basis for discrimination.
- Genotyping: Analysis is typically performed via Capillary Electrophoresis (CE). The data output is an electrophoretogram showing peaks corresponding to alleles of different lengths [14].
- Bioinformatic Simplicity: Software for STR analysis is highly mature. Genotype calling is primarily based on fragment size analysis relative to an allelic ladder. The main bioinformatic challenges involve detecting and filtering PCR artefacts like stutter peaks and interpreting peak heights in mixtures [14].
Deletion/Insertion Polymorphism-STRs (DIP-STRs)
- Principle: A DIP-STR is a haplotype combining a biallelic DIP (a "Deletion" S-allele or "Insertion" L-allele) with a closely linked, polymorphic STR [1] [4].
- Genotyping: The power of this marker lies in its allele-specific PCR design. Separate primers are designed to amplify either the S- or L-DIP allele. In an unbalanced mixture, if the major contributor is homozygous for one DIP allele (e.g., SS), primers targeting the opposite allele (e.g., L) will selectively amplify the minor contributor's DNA, provided they possess that allele. The linked STR is then genotyped to provide high discrimination power [1] [4].
- Bioinformatic Complexity: Analysis requires not only STR fragment analysis but also the interpretation of haplotype informativeness. The bioinformatic pipeline must determine if a genotype is "informative" (i.e., capable of revealing the minor donor) based on the DIP mismatch between the contributors [1].
Table 1: Core Technological Comparison of STR and DIP-STR Markers
| Feature | STR Markers | DIP-STR Markers |
|---|---|---|
| Polymorphism Type | Length (Multi-allelic) | Haplotype (Biallelic DIP + Multi-allelic STR) |
| Primary Analysis Platform | Capillary Electrophoresis (CE) | Capillary Electrophoresis (CE) |
| Key Bioinformatic Task | Fragment length analysis, stutter filtering | Haplotype calling, informativeness assessment |
| Inherent Stutter | High, requires filtering | Low or absent [9] |
| Inherent Mixture Resolution | Low for highly unbalanced mixtures (<1:10) | High (up to 1:1000 minor:major ratio) [9] [4] |
Experimental Protocols and Workflow Comparison
The experimental journey from sample to profile differs significantly between the two marker types, with corresponding implications for the bioinformatic workflow.
STR Analysis Protocol
The protocol for STR analysis is standardized and widely implemented in forensic laboratories globally.
- DNA Extraction & Quantification: Isolate DNA and measure its concentration.
- PCR Amplification: Amplify 20+ core STR loci simultaneously using a commercial multiplex kit (e.g., GlobalFiler, PowerPlex) [14].
- Capillary Electrophoresis: Separate amplified fragments by size.
- Data Analysis (Bioinformatic Pipeline):
- Fragment Sizing: Software (e.g., GeneMapper) compares sample peaks to an allelic ladder to assign allele calls.
- Artefact Filtering: Algorithms identify and filter stutter peaks, pull-up, and other noise.
- Genotype Interpretation: For mixtures, probabilistic genotyping software may be used to deconvolute contributor profiles based on peak heights and areas [28].
DIP-STR Analysis Protocol
The DIP-STR protocol is more specialized, focusing on targeting the minor DNA.
- DNA Extraction & Quantification.
- Selection of Informative Markers (Bioinformatic Pre-Screening): If reference profiles are available, bioinformatic analysis can pre-select DIP-STR markers where the major and minor contributors have opposite DIP alleles (e.g., major is SS, minor is SL or LL) [1]. Without references, multiple markers must be tested.
- Allele-Specific PCR Amplification: PCR is run with primers specific to the S or L DIP allele.
- Capillary Electrophoresis.
- Data Analysis (Bioinformatic Pipeline):
- Haplotype Calling: Software must call the DIP-STR haplotype (e.g., L-11, S-14).
- Informativeness Assessment: The pipeline determines if the result is informative for minor DNA detection based on the called genotypes [1] [4].
- Statistical Weighting: The product rule is applied across multiple informative DIP-STRs to calculate the statistical weight of the evidence [9].
The following workflow diagram visualizes the key decision points in the DIP-STR bioinformatic pipeline, particularly the critical step of assessing genotype informativeness.
Performance and Experimental Data Comparison
Experimental data from validation studies highlight the distinct performance characteristics of STR and DIP-STR markers.
Performance in Unbalanced Mixtures
A theoretical comparison using simulated mixtures demonstrated that while STRs are preferable for moderately unbalanced mixtures (up to 1:10), DIP-STRs are superior for extremely unbalanced mixtures (beyond 1:10) [2]. In practical tests on "touch" DNA samples, a set of six DIP-STRs detected a minor contributor of any sex in 54 out of 71 traces, whereas Y-STRs could only be applied to 14 of the 71 traces (all male-minor/female-major) and showed a similar detection sensitivity within that subset [24].
Analysis of Degraded DNA
The performance of STRs is highly dependent on amplicon size. Degraded DNA, which is fragmented, often leads to allele dropout in larger STR loci. DIP-STRs and other markers like mini-STRs are designed with short amplicon sizes to combat this. A new panel of 30 DIP-STRs has an average amplicon size of 230 bp, making them highly sensitive for low-quantity and degraded DNA, down to 0.06 ng, and capable of targeting a minor component contributing only 0.1% to a mixed trace [9].
Table 2: Comparative Experimental Performance Data from Key Studies
| Performance Metric | STR Markers | DIP-STR Markers | Experimental Context |
|---|---|---|---|
| Detection Sensitivity (Minor:Major Ratio) | ~1:10 (5-10% minor) [2] [4] | Up to 1:1000 (0.1% minor) [9] [4] | Simulation and controlled mixture studies |
| Success Rate on Touch DNA | Low (few minor alleles detected) [24] | High (76%, 54/71 traces) [24] | Analysis of 71 simulated two-source contact traces |
| Typing Accuracy | High, but susceptible to stutter artefacts | High, with low or no stutter [9] | Standard genotyping protocols |
| Applicability | All mixtures | All mixtures, regardless of contributor sex [24] | Forensic casework simulation |
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table details key reagents and materials essential for conducting research and developing assays with DIP-STR and STR markers.
Table 3: Essential Research Reagent Solutions for Forensic Marker Analysis
| Item | Function/Description | Application in STRs | Application in DIP-STRs |
|---|---|---|---|
| Commercial STR Multiplex Kits (e.g., GlobalFiler, PowerPlex) | Pre-optimized PCR mixes for simultaneous amplification of core STR loci. | Essential for standard forensic profiling. | Not used. |
| Allele-Specific Primers | PCR primers designed to bind selectively to one version of a polymorphic site. | Not typically used. | Critical for targeting S or L DIP alleles in a mixture [1]. |
| Thermostable DNA Polymerase | Enzyme for PCR amplification, often with "Hot Start" capability. | Required. | Required. |
| Capillary Electrophoresis System (e.g., ABI 3500) | Instrument for separating DNA fragments by size and detecting fluorescent labels. | Standard for data generation. | Standard for data generation. |
| Population Genome Databases (e.g., gnomAD, 1000 Genomes) | Public repositories of human genetic variation. | Used for frequency data. | Crucial for selecting polymorphic DIP-STR candidates and calculating haplotype frequencies [1] [9]. |
| Bioinformatic Tools for Haplotype Analysis | Custom or commercial software for calling DIP-STR haplotypes and assessing informativeness. | Not required. | Essential for data interpretation [1]. |
The choice between STR and DIP-STR markers, and their corresponding bioinformatic pipelines, is dictated by the nature of the forensic evidence. STRs, with their straightforward fragment analysis bioinformatics, remain the powerful and efficient standard for single-source or balanced mixtures. However, for the critical challenge of unbalanced DNA mixtures, DIP-STRs offer a theoretically and experimentally validated superior solution. Their bioinformatic pipeline, while more complex due to the need for haplotype calling and informativeness assessment, enables the detection of a minor contributor at ratios as low as 1:1000, irrespective of sex. The ongoing development of larger, optimized DIP-STR multiplex panels [9] and their compatibility with CE platforms ensure that this powerful technology can be integrated into forensic workflows to extract invaluable investigative leads from what were previously considered non-informative samples.
The field of forensic genetics is in a continuous state of advancement, driven by the need to analyze increasingly complex biological evidence. While Short Tandem Repeat (STR) markers have served as the gold standard for forensic identification for decades, they present significant limitations in analyzing challenging samples, particularly unbalanced DNA mixtures where one contributor's DNA is substantially more abundant than others. In standard forensic analysis, STR markers typically fail to detect the minor contributor in mixtures with ratios more extreme than 1:10, creating an analytical gap in cases such as sexual assaults where the perpetrator's DNA may be present in minimal quantities [4] [2].
To address these limitations, novel marker systems have emerged, including Deletion/Insertion Polymorphisms (DIPs) and compound markers like DIP-STRs. These markers offer enhanced sensitivity for specific forensic scenarios, particularly when combined with optimized panel selection strategies tailored to target populations. The comparative analysis of these marker technologies reveals a complex tradeoff between sensitivity, discrimination power, and practical applicability, with the optimal choice heavily dependent on the specific evidentiary context and population characteristics [2] [6].
This guide provides a comprehensive comparison of DIP panels and traditional STR markers, focusing on their relative performance characteristics, experimental applications, and implementation considerations for forensic researchers and DNA analysis professionals.
Marker Technologies: Principles and Characteristics
Short Tandem Repeat (STR) Markers
STR markers analyze regions of the genome containing tandemly repeated nucleotide sequences typically 2-6 base pairs in length. These markers are highly polymorphic due to variations in the number of repeat units, providing excellent discriminatory power for individual identification. The analysis of STR profiles relies on PCR amplification followed by capillary electrophoresis, a well-established methodology in forensic laboratories worldwide [49]. However, a significant limitation of STR analysis emerges in mixed DNA samples where the contributions are highly unbalanced. In such scenarios, the major contributor's profile may completely "mask" that of the minor contributor, with the detection threshold for the minor component typically falling between 10-20% of the total DNA [4] [2].
The statistical interpretation of STR matches incorporates population genetic parameters, particularly FST values, which quantify genetic variation among subpopulations. These parameters account for the fact that allele frequencies differ across populations, affecting match probability calculations. Higher FST values indicate greater population differentiation, necessitating more conservative match probabilities when the specific subpopulation of the donor is unknown [49] [50].
Deletion/Insertion Polymorphism (DIP) Markers
DIPs, also known as insertion-deletion polymorphisms, represent bi-allelic markers characterized by the presence or absence of specific DNA sequences. These markers typically involve sequence variations ranging from 1 to 20 base pairs. DIPs offer several advantages for forensic analysis, including: low mutation rates (estimated at approximately 10⁻⁸), absence of stutter artifacts during analysis, and compatibility with standard capillary electrophoresis platforms [6].
While individual DIP markers are less polymorphic than STRs due to their biallelic nature, this limitation is overcome by analyzing multiple DIP markers simultaneously. When strategically selected to maximize population differentiation, DIP panels can provide both individual identification capabilities and biogeographic ancestry information. The combination of DIPs with other marker types creates compound markers with enhanced properties for specific applications [6].
Compound DIP-STR Markers
DIP-STR markers represent an innovative approach to analyzing unbalanced DNA mixtures. These compound markers consist of a DIP located in close proximity (within 500 bp) to an STR region. This configuration enables a novel analysis strategy wherein the DIP component serves as an "anchor" for specific amplification of the minor contributor's DNA [4].
The analytical power of DIP-STR markers stems from their design: by designing primers that target the specific DIP allele absent in the major contributor's profile, analysts can selectively amplify the minor contributor's DNA, effectively filtering out the major contributor's genetic material. This approach enables the detection of minor DNA components in ratios as extreme as 1:1,000, dramatically improving upon the 1:10 limitation of conventional STR analysis [4] [2].
Table 1: Comparative Characteristics of Forensic DNA Marker Types
| Characteristic | STR Markers | DIP Markers | DIP-STR Markers |
|---|---|---|---|
| Polymorphism Type | Length polymorphism (repeat variations) | Presence/absence of sequences | Compound: DIP + STR |
| Allelic Variants | Multiple alleles per locus | Typically bi-allelic | Multiple alleles possible |
| Mutation Rate | ~10⁻³ | ~10⁻⁸ | Intermediate |
| Mixture Analysis | Limited (1:10 minor detection) | Limited as standalone | Excellent (up to 1:1000) |
| Ancestry Information | Limited with standard panels | Excellent with AIM-DIPs | Limited |
| Analysis Platform | Capillary electrophoresis | Capillary electrophoresis or NGS | Capillary electrophoresis |
| Stutter Artifacts | Present | Absent | STR component has stutter |
Performance Comparison and Experimental Data
Analysis of Unbalanced DNA Mixtures
The performance differential between marker systems is most pronounced in the context of unbalanced DNA mixtures. Experimental comparisons demonstrate that DIP-STR markers significantly outperform both standard STRs and Y-STRs in detecting minor DNA components in extremely unbalanced mixtures [2].
In simulated mixture experiments, DIP-STR markers successfully generated complete minor contributor profiles at ratios of 1:1000, whereas standard STR analysis failed to detect any minor contributor alleles at this extreme imbalance. Even at more moderate ratios of 1:100, STR analysis produced only partial profiles of the minor contributor, while DIP-STR markers continued to yield complete genotypes. This enhanced sensitivity stems from the allele-specific amplification strategy employed in DIP-STR analysis, which preferentially amplifies the minor contributor's DNA based on DIP allele mismatches between contributors [4].
For female-male mixtures, Y-STR markers represent the traditional solution for detecting male minor contributors. However, comparative studies reveal that DIP-STR markers provide comparable or superior performance to Y-STRs in such scenarios, with the added advantage of being applicable regardless of the sex combination of contributors [2].
Population Differentiation and Ancestry Inference
DIP markers excel in ancestry inference applications when selected as Ancestry Informative Markers (AIMs). These markers are chosen based on significant allele frequency differentials between population groups. For example, a recently developed 60-marker DIP panel demonstrated strong differentiation of East Asian populations, with pairwise FST values exceeding 0.2 between northern and southern subgroups [6].
The population differentiation capacity of DIP markers contrasts with traditional STR panels, which were primarily selected for high individual discrimination rather than ancestry information. While specialized STR panels can provide population information, DIP-based panels offer advantages in marker stability (lower mutation rates) and analysis simplicity (no stutter artifacts) for ancestry applications [6].
Table 2: Performance Metrics of a 60-Marker DIP Panel for East Asian Populations
| Performance Metric | Value | Interpretation |
|---|---|---|
| Combined Probability of Discrimination | 0.999999999999 | Extremely high power for individual identification |
| Cumulative Probability of Paternity Exclusion | 0.9937 | High effectiveness in relationship testing |
| Marker Stability | Mutation rate ~10⁻⁸ | Highly stable across generations |
| Ancestry Differentiation | FST > 0.2 between subgroups | Effective for distinguishing East Asian subpopulations |
| Amplicon Size Range | <200 bp | Suitable for degraded DNA samples |
Statistical Weight of Evidence
The forensic utility of any genetic marker system ultimately depends on its statistical power for individual identification. Likelihood Ratio (LR) simulations comparing DIP-STRs with standard STRs reveal method-specific advantages depending on the mixture scenario [2].
For moderately unbalanced mixtures (ratios up to 1:10), standard STR markers generally yield higher LRs due to the greater polymorphism of STR loci and the availability of more extensive population databases. However, in extremely unbalanced mixtures (exceeding 1:10), DIP-STR markers provide substantially stronger statistical support, as they can generate complete profiles where STRs fail entirely [2].
This performance differential highlights the context-dependent nature of marker selection: DIP-STRs offer unparalleled sensitivity for trace DNA analysis in mixtures, while standard STRs maintain advantages in conventional single-source or balanced mixture analysis.
Panel Selection Strategies and Methodologies
Marker Selection Algorithms
The optimization of marker panels for specific populations employs sophisticated computational approaches that move beyond simple univariate selection of the most informative individual markers. Comparative studies of algorithm performance across multiple species (including humans) have demonstrated that multivariate approaches generally outperform simple marker ranking methods [51].
The "greedy" algorithm sequentially selects markers that provide the greatest improvement in assignment accuracy when combined with previously selected markers, often including loci that would be overlooked by univariate methods. The "maximin" algorithm selects markers that maximize the minimum genetic distance between populations, ensuring robust differentiation across all population pairs. In human population assignment, these advanced algorithms can achieve over 94% accuracy with 13-16 optimally selected markers, compared to approximately 80% accuracy with randomly selected markers [51].
Population-Specific FST Considerations
The interpretation of matching DNA profiles requires careful consideration of population genetic structure, quantified by FST values. This parameter measures genetic differentiation among subpopulations and significantly impacts match probability calculations [49] [50].
Recent analyses of global STR data from 446 populations reveal that FST values exhibit substantial variation across populations and marker sets, suggesting that conservative theta values (0.01-0.03) commonly used in forensic calculations may not adequately account for population structure in all cases. This finding has particular relevance for admixed populations or cases where the ancestral background of the donor is unknown, necessitating careful marker selection and appropriate reference population databases [50].
Implementation in Multiplex Assays
The practical implementation of optimized marker panels requires their integration into efficient multiplex PCR assays compatible with standard forensic workflows. Recent validation studies have demonstrated successful multiplexing of up to 90 microhaplotype markers (a related marker type) in single-tube reactions, with amplicon sizes kept under 200 bp to facilitate analysis of degraded DNA [52].
Similar principles apply to DIP panel design, with the 60-marker East Asian panel serving as an exemplar of optimized multiplex development. This panel incorporates 56 autosomal DIPs, 3 Y-chromosome DIPs, and the Amelogenin sex marker in a single multiplex assay, demonstrating the feasibility of high-multiplex DIP panels for comprehensive forensic analysis [6].
Experimental Protocols and Workflows
DIP-STR Analysis Protocol
The analytical workflow for DIP-STR markers employs a targeted amplification approach that leverages the compound nature of these markers to selectively amplify the minor contributor's DNA [4].
Sample Preparation and DNA Extraction
- Extract DNA from forensic samples using standard silica-based methods
- Quantify DNA using fluorescent quantification methods (e.g., qPCR)
- Normalize samples to working concentrations (typically 0.1-1 ng/μL)
PCR Amplification
- Design DIP-STR primers with the 3' end targeting the DIP allele of interest
- Include fluorescent labels for downstream fragment analysis
- Use touchdown PCR protocols to enhance specificity:
- Initial denaturation: 94°C for 2 minutes
- 10 cycles of: 94°C for 30s, 65°C (-1°C/cycle) for 30s, 72°C for 45s
- 25 cycles of: 94°C for 30s, 55°C for 30s, 72°C for 45s
- Final extension: 72°C for 10 minutes
Capillary Electrophoresis and Analysis
- Separate PCR products using capillary electrophoresis systems
- Analyze data with genotyping software to determine allele sizes
- Interpret results based on DIP-STR haplotypes
This protocol capitalizes on the principle that primers matching the DIP allele absent in the major profile will preferentially amplify the minor contributor's DNA, effectively filtering the mixture at the amplification stage [4].
DIP Panel Validation Protocol
The development and validation of population-specific DIP panels follows rigorous guidelines established by the Scientific Working Group on DNA Analysis Methods (SWGDAM) [6].
Marker Selection and Panel Design
- Identify candidate DIPs from 1000 Genomes Project and dbSNP databases
- Apply selection criteria: MAF ≥ 0.1, length variation 1-20 bp, chromosomal distribution
- Evaluate population differentiation capacity using FST metrics
- Design primers with amplicons <200 bp for degraded DNA compatibility
Multiplex Assay Optimization
- Test primer combinations using software tools (e.g., AutoDimer)
- Optimize PCR conditions through fractional factorial designs:
- Magnesium concentration gradient (1.5-3.5 mM)
- Primer mix concentration (0.5× to 1.5×)
- Annealing temperature gradient (55-65°C)
- Cycle number optimization (21-27 cycles)
- Validate specificity with diverse population samples
Performance Validation
- Sensitivity: Test with serial DNA dilutions (0.1-10 ng)
- Species specificity: Evaluate cross-reactivity with non-human DNA
- Stability: Assess performance with degraded and inhibited samples
- Mixture analysis: Determine detection limits in mixed samples
- Reproducibility: Inter-laboratory and intra-laboratory testing
Population Studies
- Genotype diverse population samples (n≥100 per population)
- Calculate forensic parameters: PD, PE, CPD, CPE
- Evaluate ancestry inference: PCA, STRUCTURE, phylogenetic analysis
This comprehensive validation ensures that DIP panels meet forensic standards for reliability, reproducibility, and statistical power [6].
Essential Research Reagents and Materials
Table 3: Essential Research Reagents for Forensic Marker Panel Development
| Reagent/Material | Function | Application Examples |
|---|---|---|
| Silica-based DNA Extraction Kits | Isolation of high-quality DNA from various forensic samples | Organic extraction alternatives for blood, saliva, touch DNA |
| Quantitative PCR (qPCR) Kits | Accurate DNA quantification and quality assessment | Human-specific quantification, degradation assessment |
| PCR Master Mixes | Amplification of target markers with high fidelity | Multiplex PCR optimization with balanced amplification |
| Fluorescent Dye-labeled Primers | Detection of amplified fragments during capillary electrophoresis | STR, DIP, and DIP-STR multiplex panels |
| Capillary Electrophoresis Polymers | Size separation of fluorescently labeled PCR fragments | Allele sizing and genotyping on ABI platforms |
| Population Reference Samples | Database development and allele frequency estimation | Population-specific marker validation (1000 Genomes) |
| Statistical Genetics Software | Data analysis, population genetics parameters | FST calculation, PCA, STRUCTURE analysis |
| Multiplex Design Tools | In silico primer evaluation and panel optimization | AutoDimer, Primer Premier, multiplex manager |
The comparative analysis of DIP panels and STR markers reveals a nuanced landscape where each technology offers distinct advantages for specific forensic scenarios. STR markers maintain their position as the gold standard for conventional single-source and moderately mixed samples, benefiting from extensive population databases and established interpretation frameworks. DIP-based panels excel in ancestry inference and, when configured as DIP-STR compound markers, provide unprecedented sensitivity for analyzing extremely unbalanced mixtures [2] [6].
The optimal selection of informative markers for specific populations requires consideration of multiple factors: the evidentiary context (mixture complexity, DNA quality), population characteristics (genetic diversity, substructure), and analytical requirements (sensitivity, discrimination power). Advanced selection algorithms that employ multivariate approaches consistently outperform simple ranking methods, enabling the development of highly optimized panels with minimal marker counts [51].
Future directions in forensic panel optimization will likely involve the integration of multiple marker types (STRs, DIPs, SNPs, microhaplotypes) in unified multiplex assays, leveraging the complementary strengths of each system. The ongoing development of population-specific reference databases will further enhance the statistical power and applicability of these panels across diverse global populations. As these technologies mature, they will continue to expand the boundaries of forensic DNA analysis, enabling reliable genotyping from increasingly challenging and complex biological evidence [6] [52].
Performance Metrics: A Side-by-Side Evaluation of Efficacy
In forensic genetics and medical diagnostics, the analysis of mixed DNA samples presents a significant challenge, particularly when the contributors are present in highly unbalanced proportions. Such scenarios are common in forensic casework, including sexual assault evidence where a perpetrator's trace DNA is mixed with a large quantity of the victim's DNA, or in prenatal testing where fetal DNA is found in maternal plasma amid a high background of maternal DNA [4] [1]. Standard forensic markers, namely Short Tandem Repeats (STRs), encounter substantial limitations in these contexts due to PCR amplification bias, typically failing to detect a minor contributor when it represents less than 5-10% of the total DNA [4] [2]. This sensitivity gap means that potentially crucial evidence remains undetected. To address this analytical frontier, scientists have developed advanced marker systems capable of deconvoluting mixtures at ratios as extreme as 1:1000. This comparison guide provides an objective analysis of these technologies, focusing on their operational principles, experimental performance, and practical applications for researchers and forensic professionals.
Competing Technologies for Extreme Mixture Deconvolution
Three principal marker technologies have emerged for analyzing unbalanced DNA mixtures: DIP-STRs, Y-STRs, and SNP-STRs. Each operates on a distinct genetic principle and offers different advantages and limitations.
- DIP-STR Markers: A compound marker system that pairs a Deletion/Insertion Polymorphism (DIP) with a closely linked STR [4]. The core innovation is an allele-specific amplification approach where one PCR primer is designed to overlap the DIP region, specifically targeting either the insertion (L) or deletion (S) allele. This allows selective amplification of the minor DNA component when it possesses a DIP allele not shared by the major contributor, while the linked STR provides high discrimination power [48] [1].
- Y-STR Markers: Short Tandem Repeats located on the Y chromosome. Their primary advantage is the inherent ability to target male DNA in a mixture with a high female DNA background, as female DNA lacks the Y chromosome entirely [1] [24].
- SNP-STR Markers: Another compound marker system, which links a Single Nucleotide Polymorphism (SNP) with a nearby STR. Similar to DIP-STRs, it uses an Amplification Refractory Mutation System (ARMS) PCR with allele-specific primers for the SNP to selectively amplify the minor DNA component [1] [53].
The table below summarizes the fundamental characteristics of these systems.
| Feature | DIP-STR | Y-STR | SNP-STR |
|---|---|---|---|
| Genetic Basis | Compound DIP + STR haplotype [4] | Y-Chromosome STRs [1] | Compound SNP + STR haplotype [1] |
| Targeting Principle | Allele-specific PCR based on DIP variant [48] | Chromosome-specific (Y) amplification [24] | Allele-specific PCR based on SNP variant [53] |
| Applicability | Any two-person mixture, regardless of sex [24] | Only male minor in female major mixtures [2] | Any two-person mixture, regardless of sex [1] |
| Statistical Independence | Independent markers across genome [48] | Single haplotype; product rule invalid [4] | Independent markers across genome [1] |
DIP-STR Mechanism: A Closer Look
The following diagram illustrates the allele-specific amplification mechanism that enables DIP-STRs to target a minor DNA contributor in an extreme mixture.
Diagram 1: DIP-STR Allele-Specific Amplification. The 'S' primer fails to amplify the minor DNA's 'L' allele, while the 'L' primer selectively amplifies the minor contributor's DNA, enabling its detection despite a 1000-fold excess of major DNA [4] [48].
Performance Comparison: Quantitative Data
The theoretical advantages of different markers must be validated through rigorous experimental testing. The following table consolidates key performance metrics from empirical studies for DIP-STRs, Y-STRs, and SNP-STRs, providing a direct comparison of their capabilities.
Table 2: Experimental Performance Comparison of Marker Systems for Unbalanced Mixtures.
| Performance Metric | DIP-STR | Y-STR | SNP-STR |
|---|---|---|---|
| Max Reported Sensitivity (Minor:Major) | 1:1,000 [4] [48] | ~1:100 to 1:1,000 (female:male) [1] [24] | ~1:40 to 1:1,000 [1] [53] |
| Minimum DNA Template | 0.03 - 0.1 ng (minor) [48] | Varies by kit/protocol | 0.025 - 0.05 ng (minor) [53] |
| Informativeness Rate | ~50-75% (on simulated touch DNA) [24] | 100% (for male:female mixtures) [24] | Requires population data for selection [1] |
| Best Application Context | Extremely unbalanced mixtures, any sex combination [2] [24] | Male-specific detection in sexual assault cases [1] | Degraded and unbalanced mixtures (short amplicons) [53] |
Experimental Protocols and Methodologies
To ensure reproducibility and facilitate adoption, detailed methodologies for the leading DIP-STR technology are outlined below.
DIP-STR Marker Selection and Design
The development of a forensic-grade DIP-STR set follows stringent criteria [48]:
- Database Mining: Candidate regions are identified from genomic databases (e.g., UCSC Genome Browser) by locating DIPs within 200 base pairs of a known STR.
- Feature Filtering: Selected DIP-STRs must be located in non-coding regions on different chromosomes to ensure independent inheritance and avoid linkage with phenotypic traits. DIPs should have a minor allele frequency (MAF) > 0.2 to increase the probability of informative alleles.
- Forensic Optimization: For modern sets, amplicon size is kept short (< 300 bp) to analyze degraded DNA. The linked STRs should be tri-, tetra-, or penta-nucleotide repeats to minimize stutter artifacts common with di-nucleotide repeats [48].
Detailed DIP-STR Genotyping Workflow
The experimental protocol for analyzing a sample using DIP-STR markers involves the following key steps [4] [48]:
- DNA Extraction and Quantification: Standard forensic protocols are used to extract DNA from the mixed stain, followed by precise quantification.
- Allele-Specific PCR Setup: Separate PCR reactions are set up for the 'S' and 'L' allele-specific primers for each DIP-STR marker.
- Reaction Volume: Typically 10-25 μL.
- DNA Template: 0.1-1 ng of total DNA from the mixture.
- Thermocycling Conditions: An initial denaturation (e.g., 94°C for 5 min), followed by 25-30 cycles of denaturation (e.g., 94°C for 30 s), annealing (e.g., 60°C for 30 s), and extension (e.g., 72°C for 45 s), with a final extension (e.g., 72°C for 10-20 min).
- Capillary Electrophoresis (CE): The PCR products are separated and detected using a genetic analyzer (e.g., ABI 3500). The fluorescently labeled fragments are sized against an internal lane standard.
- Data Analysis and Interpretation: The electropherograms are analyzed. A successful detection of the minor contributor is indicated by the presence of peaks only in the PCR reaction targeting the DIP allele not possessed by the major contributor [48] [24].
The Scientist's Toolkit: Essential Research Reagents
Implementing mixture deconvolution assays requires a specific set of laboratory reagents and tools. The table below details the essential components of the research toolkit.
Table 3: Key Research Reagent Solutions for DIP-STR Analysis.
| Reagent / Tool | Function / Description | Example Specifications / Notes |
|---|---|---|
| Allele-Specific Primers | Core reagent for targeted amplification; one primer overlaps DIP, other is downstream of STR [4]. | HPLC-purified; 5' fluorescent dye label (e.g., 6-FAM, VIC). |
| High-Fidelity DNA Polymerase | Enzymatic amplification of target DIP-STR loci with low error rate. | Hot-start Taq polymerase to prevent non-specific amplification. |
| Genetic Analyzer | Instrument for fragment analysis by capillary electrophoresis. | ABI 3500 series; required for high-resolution sizing of STR alleles [48]. |
| Population DNA Datasets | Essential for calculating allele frequencies and match probabilities [1] [7]. | 1000 Genomes Project; ALFRED Database; in-house population samples. |
| Commercial Quantification Kits | Precise measurement of total human DNA in a sample prior to PCR. | Qubit dsDNA HS Assay Kit; PicoGreen assay [53]. |
The direct comparison of sensitivity, applicability, and operational requirements demonstrates that no single marker system is universally superior. The choice of technology must be guided by the specific sample context and analytical question. For the most challenging forensic and diagnostic scenarios involving extremely unbalanced two-person mixtures of any sex combination, DIP-STR markers currently offer a unique combination of high sensitivity (1:1000), powerful discrimination, and practical workflow on standard capillary electrophoresis platforms [48] [24]. Meanwhile, Y-STRs remain the gold standard for sexual assault cases with a female victim and male suspect, and SNP-STRs show promise for highly degraded samples due to their shorter amplicon lengths [53].
Future research will focus on expanding multiplexing capabilities to increase the number of DIP-STRs analyzed in a single reaction, thereby improving success rates and statistical power. Furthermore, integration with Massively Parallel Sequencing (MPS) may enhance mixture deconvolution by providing deeper insights into haplotype structures and detecting additional linked polymorphisms [1]. As these technologies evolve, the ability to recover informative DNA profiles from vanishingly small amounts of material in complex mixtures will continue to transform the frontiers of forensic science and medical diagnostics.
The quantitative assessment of evidence lies at the heart of modern forensic science, with the likelihood ratio (LR) serving as the fundamental metric for expressing statistical weight in forensic genetics. This framework provides a balanced method for evaluating two competing propositions given the observed evidence [16]. In DNA analysis, the choice of genetic markers directly influences the discriminatory power of forensic testing and consequently affects the magnitude of LRs that can be obtained.
Short Tandem Repeats (STRs) have long been the gold standard in forensic DNA analysis due to their high polymorphism [54]. However, Deletion/Insertion Polymorphisms (DIPs) have emerged as powerful complementary markers with distinct advantages for specific forensic applications [6] [16]. DIPs, also known as insertion/deletion (InDel) polymorphisms, are biallelic length polymorphisms resulting from the insertion or deletion of DNA fragments throughout the human genome [54].
This guide provides a systematic comparison of the statistical weight of evidence generated by DIP panels versus traditional STR forensic markers, with a specific focus on likelihood ratio performance across different forensic applications including individual identification, kinship analysis, and ancestry inference.
Molecular Characteristics and Typing Platforms
Fundamental Genetic Properties
Understanding the core molecular differences between STR and DIP markers is essential for evaluating their performance in forensic applications.
Table 1: Comparative Genetic Properties of STR and DIP Markers
| Property | STR Markers | DIP Markers |
|---|---|---|
| Molecular Nature | Tandemly repeated sequences (2-6 bp units) | Insertion/Deletion sequences |
| Allele Variability | Multi-allelic (highly polymorphic) | Biallelic (insertion/deletion) |
| Mutation Rate | Relatively high (~10⁻³) | Lower (~10⁻⁸) [6] |
| Stutter Artifacts | Present (PCR artifact) | Absent [6] [16] |
| Amplicon Size | Typically longer fragments | Can be designed as short fragments (<200 bp) [16] |
| Genotyping Platform | Capillary Electrophoresis (CE) | Capillary Electrophoresis (CE) [54] |
Compound Marker Systems
To overcome the limitations of biallelic DIP markers, researchers have developed compound marker systems that enhance polymorphism:
DIP-STR Markers: These novel analytical approaches pair deletion–insertion polymorphisms (DIP) with nearby STR markers, allowing unambiguous genotyping of a minor component in the presence of a major component at ratios up to 1:1,000 [4]. The compound nature generates high-level polymorphism suitable for identity testing.
Multi-InDel Markers: These consist of two or more closely linked InDel markers forming a compound marker that offers the advantages of microhaplotypes and can be genotyped using capillary electrophoresis platforms [54]. Each Multi-InDel marker can yield four alleles (0, 1, 2, 3) based on the combination of insertions and deletions at the two component loci.
Figure 1: DIP-STR Compound Marker Concept. This diagram illustrates how pairing DIP with STR markers enables minor component genotyping in highly unbalanced DNA mixtures.
Forensic Efficiency Parameters and Likelihood Ratio Comparisons
Individual Identification Power
The discrimination power of forensic markers is quantified through several key parameters, with combined probability of discrimination (CPD) representing the overall system effectiveness for individual identification.
Table 2: Forensic Efficiency Parameters for Individual Identification
| Marker System | Population | CPD Value | CPM Value | Reference |
|---|---|---|---|---|
| 43 A-DIP Panel | Chinese Yi | 0.99999999999999999988 | 1.11433E-18 | [16] |
| 43 A-DIP Panel | Chinese Hani | 0.99999999999999999991 | 8.24299E-19 | [16] |
| 43 A-DIP Panel | Chinese Miao | 0.99999999999999999979 | 4.21721E-18 | [16] |
| 41 Multi-InDel Panel | Chinese Kazakh | 0.99999999999999999999999984 | - | [54] |
| 41 Multi-InDel Panel | Chinese Kyrgyz | 0.99999999999999999999999972 | - | [54] |
| 60 DIP Panel | East Asian | 0.999999999999 | - | [6] |
The exceptionally high CPD values demonstrated by multi-InDel panels highlight their remarkable power for individual identification, exceeding standard STR systems in discrimination capability for the populations tested.
Kinship Analysis Performance
Kinship testing represents one of the most forensically challenging applications, requiring high-resolution genetic data to distinguish related from unrelated individuals.
Table 3: Kinship Analysis Performance Metrics
| Marker System | Population | CPE Value | Full Sibling Discrimination Rate | Reference |
|---|---|---|---|---|
| 43 A-DIP Panel | Chinese Yi | 0.999610217 | 96.65% (average across 3 groups) | [16] |
| 43 A-DIP Panel | Chinese Hani | 0.999629285 | - | [16] |
| 43 A-DIP Panel | Chinese Miao | 0.999582084 | - | [16] |
| 41 Multi-InDel Panel | Chinese Kazakh | 0.999998887 | 98.82% (average for FS pairs) | [54] |
| 41 Multi-InDel Panel | Chinese Kyrgyz | 0.999999349 | - | [54] |
| 60 DIP Panel | East Asian | 0.9937 | - | [6] |
The likelihood ratio method has been proven more accurate than identity by state (IBS) methods for kinship testing with biallelic markers such as DIPs [16]. For the 43 A-DIP panel, simulation studies demonstrated that an average of 96.65% of full sibling pairs could be distinguished from unrelated individual pairs (LR > 1), with an average false positive rate of 3.69% across three Yunnan population groups [16].
Figure 2: Likelihood Ratio Framework for Kinship Analysis. This diagram illustrates the logical relationship between competing hypotheses and the interpretation of likelihood ratios in kinship testing.
Experimental Protocols and Methodologies
Multiplex PCR Amplification and Capillary Electrophoresis
The experimental workflow for DIP analysis follows standardized protocols compatible with existing forensic laboratory infrastructure:
Sample Preparation and DNA Extraction: Blood samples are collected on FTA cards and dried before extraction. Genomic DNA is extracted using the Chelex-100 method, with 9947A, 9948, and nuclease-free water serving as positive and negative controls, respectively [16]. DNA concentration and purity are determined using a NanoDrop 1000 spectrophotometer.
Primer Design and Panel Construction: Primers are designed using Primer Premier 5.0 and evaluated with AutoDimer software. Markers are selected based on specific criteria including minimum allele frequency (MAF ≥ 0.1), allele length variation (1-20 bp), and independent inheritance (located on different chromosomes or >5 Mb apart on the same chromosomal arm) [6]. The amplicon sizes are typically limited to <200 bp to facilitate analysis of degraded DNA.
Multiplex PCR Amplification: Amplification is performed using a GeneAmp PCR System 9700 thermal cycler. Typical reaction volumes are 10 μL, consisting of 2 μL 2.0× master mix, 1 μL (1 ng) template DNA, 2 μL 1.0× primer mix, and 5 μL nuclease-free water [54]. Optimal cycling conditions include initial denaturation at 94°C, followed by 25 cycles of denaturation at 94°C, annealing at 60°C, and extension at 72°C, with a final extension at 60°C.
Capillary Electrophoresis and Genotyping: Amplified products are separated and detected using a 3500xL Genetic Analyzer. DNA profiles are analyzed using GeneMapper ID-X software, with genotypes determined based on fragment sizes. For Multi-InDel markers, genotypes are assigned as allele 0 (deletion at both loci), allele 3 (insertion at both loci), or alleles 1/2 (mixed insertion/deletion) [54].
Data Analysis and Statistical Calculations
Forensic Parameters Calculation: Allele frequencies and forensic statistical parameters including typical paternity index (TPI), power of exclusion (PE), polymorphic information content (PIC), match probability (MP), and power of discrimination (PD) are calculated using STRAF (STR Analysis for Forensic) online tool [6] [16]. Combined probability of discrimination (CPD) is calculated as CPD = 1 - Π(1 - DPᵢ), and cumulative probability of exclusion (CPE) as CPE = 1 - Π(1 - PEᵢ).
Population Genetic Analyses: Hardy-Weinberg equilibrium (HWE) and linkage disequilibrium (LD) are tested using GENEPOP 4.0 or Arlequin software, with p-values adjusted using Bonferroni correction [6] [16]. Population genetic analyses include principal component analysis (PCA), STRUCTURE analysis, phylogenetic tree construction, and pairwise FST calculations to evaluate population differentiation.
Likelihood Ratio Calculations for Kinship: For kinship analysis, likelihood ratios are calculated using the Familias 3 software [54]. The prosecution hypothesis (Hp) posits that two individuals are related (full siblings or half siblings), while the defense hypothesis (Hd) posits that they are unrelated. LR distributions for different relationships are visualized using R software, with 10,000 simulated pairs for each relationship category.
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Materials and Reagents for DIP-Based Forensic Analysis
| Item | Function | Example Products/Protocols |
|---|---|---|
| FTA Cards | Biological sample collection and preservation | Whatman FTA Cards |
| Chelex-100 | DNA extraction from bloodstains | Chelex-100 protocol [54] |
| NanoDrop Spectrophotometer | DNA quantification and purity assessment | Thermo Scientific NanoDrop 1000 |
| Multiplex PCR Master Mix | Simultaneous amplification of multiple loci | 2.0× master mix [54] |
| Fluorescently-labeled Primers | Target amplification with detection capability | 6-FAM, HEX, TAMRA, ROX dyes [16] |
| Thermal Cycler | DNA amplification with precise temperature control | GeneAmp PCR System 9700 |
| Capillary Electrophoresis System | Fragment separation and detection | ABI 3500xL Genetic Analyzer |
| Internal Size Standard | Accurate fragment size determination | OG 500 (Microread Genetics) |
| Genotyping Software | Automated allele calling and data analysis | GeneMapper ID-X Software |
| Population Genetics Software | Statistical analysis and population comparisons | STRAF, GENEPOP, STRUCTURE, Familias 3 |
Performance in Challenging Forensic Scenarios
Analysis of Degraded and Mixed Samples
DIP markers demonstrate particular advantages in forensic scenarios involving compromised samples:
Degraded DNA Analysis: The shorter amplicon sizes achievable with DIP markers (<200 bp) make them particularly suitable for analyzing degraded DNA samples commonly encountered in forensic casework [54] [16]. Validation studies of the 60 DIP panel demonstrated robust performance with poor-quality samples, yielding reliable genotypes even from severely degraded DNA [6].
Unbalanced DNA Mixtures: DIP-STR markers show exceptional performance in resolving highly unbalanced DNA mixtures, successfully genotyping minor components at ratios up to 1:1,000 [4]. This capability is particularly valuable for analyzing mixed stains in forensic investigations and detecting DNA microchimerism in medical diagnostics, where standard STR markers typically require the minor component to represent at least 10-20% of the total DNA for reliable detection.
Ancestry Inference and Population Studies
DIPs serve as valuable ancestry-informative markers (AIMs) due to their significant diversity across different populations:
Biogeographical Ancestry Prediction: Machine learning algorithms, including extreme gradient boosting (XGBoost) and support vector machine (SVM), have been employed to construct prediction models for continental origins based on DIP markers [16]. Studies with 43 A-DIPs demonstrated that approximately 82.39% (95% CI 0.7984, 0.8474) of unrelated individuals could be correctly assigned to their continental origins [16].
Population Genetic Studies: DIP-based panels have proven effective in elucidating fine-scale population structures. For example, the 60 DIP panel specifically tailored for East Asian populations successfully differentiated northern and southern East Asian subgroups, providing enhanced resolution in forensic analyses [6]. Similarly, Multi-InDel analyses revealed that Chinese Kazakh and Kyrgyz groups exhibit an East Asia-Europe admixed ancestry pattern while maintaining closer genetic affinities with East Asian populations [54].
The comparative analysis of likelihood ratios and statistical weight of evidence demonstrates that DIP panels offer compelling advantages over traditional STR markers for specific forensic applications. While STRs remain the established standard for routine database generation, DIP-based systems provide enhanced performance in analyzing challenging samples, including degraded DNA and highly unbalanced mixtures.
The compound marker approaches, particularly DIP-STR and Multi-InDel systems, effectively address the limitations of biallelic DIP markers while maintaining the advantages of low mutation rates, absence of stutter artifacts, and compatibility with standard capillary electrophoresis platforms. The exceptional discrimination power demonstrated by multi-InDel panels, with CPD values often exceeding those of conventional STR systems, positions these markers as valuable tools for advancing forensic genetics.
For researchers and forensic practitioners, the selection between STR and DIP marker systems should be guided by the specific evidentiary context, sample quality, and analytical requirements. The integration of both marker types in forensic practice offers a comprehensive approach to maximizing evidential value across diverse casework scenarios.
This guide provides a comparative analysis of Deletion/Insertion Polymorphism (DIP) panels and traditional Short Tandem Repeat (STR) markers for forensic DNA analysis, focusing on validation data for sensitivity, specificity, and reproducibility.
Performance Comparison: DIP Markers vs. STRs
The table below summarizes key performance metrics from validation studies for DIP-based panels and standard STR systems.
| Parameter | DIP-based Panels | Traditional STR Markers | Key Findings |
|---|---|---|---|
| Sensitivity (Minor Contributor Detection) | Effective in ratios up to 1:1000 (DIP-STR markers) [4] [24]. | Limited to ~1:10 minor contributor detection [2] [24]. | DIP-STRs are 100x more sensitive for unbalanced mixtures [24]. |
| Analysis Specificity | Autosomal DIP-STRs work for any sex combination of contributors [24]. | Y-STRs require male minor/female major contributor profile [2] [24]. | DIP-STRs resolve a wider range of forensic scenarios [24]. |
| Typing Reproducibility | Standard deviation < 0.2 bp under optimized CE conditions [6]. | Standard deviation < 0.2 bp with capillary array electrophoresis [55]. | Both systems show high reproducibility with modern CE platforms [6] [55]. |
| Performance with Degraded DNA | Panel with amplicons ≤ 200 bp demonstrated reliable genotyping [6]. | Larger amplicon sizes (~200-500 bp) prone to dropout [14]. | Shorter DIP amplicons provide superior degradation tolerance [6] [14]. |
| Application in Touch DNA | Detected minor contributor in 54 of 71 simulated touch DNA traces [24]. | Y-STRs detected minor male in 14 of 71 traces (male-specific) [24]. | DIP-STRs significantly expand investigative leads from contact traces [24]. |
Detailed Experimental Protocols and Validation Data
Validation of a 60-Plex DIP Panel for Forensic Application
A 2025 study established a 60-multiplex DIP panel (56 autosomal DIPs, 3 Y-DIPs, and Amelogenin) for East Asian populations [6].
- Sensitivity and Specificity: The panel underwent developmental validation following SWGDAM guidelines. Tests included PCR condition optimization, sensitivity, species specificity, stability, mixture analysis, and reproducibility [6]. The panel demonstrated robust performance with degraded DNA, attributed to its short amplicons (limited to 200 bp) [6].
- Reproducibility and Accuracy: The validation study confirmed the panel's ability to yield reliable and reproducible genotypes across various forensic sample types, including case samples and degraded DNA [6].
- Discriminatory Power: The panel achieved a combined probability of discrimination (CPD) of >0.999999999999 and a cumulative probability of paternity exclusion (CPE) of 0.9937, confirming its power for personal identification [6].
DIP-STR Workflow for Unbalanced DNA Mixtures
The DIP-STR method is a compound marker designed to deconvolute extremely unbalanced two-source DNA mixtures.
DIP-STR Mixture Deconvolution Workflow
- Experimental Protocol: The method uses allele-specific PCR amplification of haplotypes formed by a DIP and a closely linked STR. Primers are designed to selectively amplify the DIP allele unique to the minor contributor, preventing amplification of the major contributor's DNA [4] [24].
- Sensitivity Benchmarking: In a study analyzing 71 simulated "touch" DNA traces, DIP-STR markers successfully detected the minor contributor in 54 traces (76%). In the subset of traces with a male minor and female major contributor, DIP-STRs showed similar sensitivity to Y-STRs but have the advantage of being applicable to any sex combination of contributors [24].
STR Genotyping Reproducibility and Limitations
- Reproducibility Protocol: A foundational study using capillary array electrophoresis demonstrated that STR genotyping can achieve a standard deviation of less than 0.2 bp under optimized conditions. This high level of precision is achieved by normalizing results to known typing controls, allowing accuracies to within 1 bp of the actual fragment size [55].
- Inherent Limitations: Standard STR profiling fails when the minor component constitutes less than 5-10% of the total DNA due to PCR amplification bias and the "masking" effect of the major contributor's alleles [2] [24]. Furthermore, Y-STRs are limited to mixtures with a specific sex mismatch [4] [2].
The Scientist's Toolkit: Key Research Reagents
The table below lists essential reagents and their functions for implementing DIP-STR and STR assays.
| Reagent / Tool | Function in Assay |
|---|---|
| Allele-Specific Primers | Selectively amplifies the DIP allele of the minor contributor in a mixture, core to DIP-STR specificity [4]. |
| Multiplex PCR Master Mix | Amplifies multiple genetic loci simultaneously; requires optimization of Mg2+ concentration and polymerase [6] [14]. |
| Fluorescent Dye Labels | Tags primers for different loci, allowing simultaneous detection and fragment size analysis via capillary electrophoresis [6] [14]. |
| Capillary Electrophoresis System | Platform for high-resolution separation and precise sizing of DNA fragments (e.g., DIP or STR amplicons) [6] [55]. |
| Allelic Ladders | Contains common alleles for each locus, serving as a reference standard for accurate genotype calling [14]. |
| Population DNA Databases | Provide allele frequency data essential for calculating the statistical weight of the evidence (e.g., 1000 Genomes Project) [6] [56]. |
Marker Selection Guide for DNA Mixtures
The analysis of DNA mixtures where contributors are present in vastly different proportions remains a significant challenge in forensic science. Standard forensic markers, particularly autosomal Short Tandem Repeats (STRs), encounter limited sensitivity in characterizing such unbalanced mixtures due to PCR amplification bias, often failing to detect a minor DNA component contributing less than 5-10% of the total DNA [4] [1] [2]. This limitation has profound implications for justice, affecting the analysis of evidence from sexual assaults, "touch" DNA, and other forensic scenarios involving trace biological material.
Two advanced approaches have emerged to address this challenge: sequencing-based STR analysis and typing using Deletion/Insertion Polymorphism (DIP) panels. Sequencing-based STR methods leverage next-generation sequencing (NGS) platforms to provide deep sequence information, while DIP panels—often designed as compound markers paired with STRs (DIP-STRs)—utilize innovative primer design to target minor DNA components with exceptional sensitivity. This guide provides a comparative analysis of these methodologies, offering forensic researchers and scientists objective performance data and experimental protocols to inform their genotyping strategies.
Sequencing-Based STR Typing
Sequencing-based STR analysis moves beyond traditional capillary electrophoresis by using next-generation sequencing platforms to determine both the length and the specific nucleotide sequence of STR alleles. This method involves library preparation from genomic DNA, target enrichment via PCR-based approaches, massively parallel sequencing, and sophisticated bioinformatic analysis for allele calling. It can simultaneously process hundreds of samples and targets, providing sequence-level variation that increases discriminatory power beyond length-based typing alone. However, challenges remain for extremely unbalanced mixtures, as the technique still faces fundamental PCR competition issues where major DNA components can overwhelm minor contributors during amplification [4] [1].
DIP Panel Typing
DIP panels represent an innovative approach specifically designed for resolving unbalanced mixtures. The DIP-STR marker, a compound genetic marker, relies on pairing a Deletion/Insertion Polymorphism (DIP) with a closely linked Short Tandem Repeat (STR) [4]. The core innovation lies in the primer design: one primer is sequence-specific, overlapping the deleted or inserted region (DIP), while the other primer anneals downstream of the adjacent STR polymorphism. This design enables allele-specific amplification, selectively targeting a minor DNA contributor when it possesses DIP alleles not shared with the major DNA [48].
The fundamental workflow for DIP-STR analysis, from DNA mixture to final genotype profile, follows a straightforward PCR and capillary electrophoresis process that is familiar to forensic laboratories.
Comparative Performance Analysis
Sensitivity in Unbalanced Mixture Resolution
The most significant performance difference between these technologies emerges in their ability to resolve extremely unbalanced DNA mixtures.
Table 1: Sensitivity Comparison for Minor DNA Detection
| Technology | Minor DNA Detection Limit | Optimal DNA Input | Key Limitation |
|---|---|---|---|
| Standard STR (Capillary Electrophoresis) | 1:10 to 1:20 ratio [1] [2] | ~1 ng total DNA [48] | PCR competition effects |
| Sequencing-Based STR | Improved over standard STR, but limited by PCR bias [4] | Platform-dependent | Remaining PCR amplification bias [4] |
| DIP-STR Panels | Up to 1:1000 ratio [4] [24] [48] | 0.03-0.1 ng of minor DNA [48] [9] | Requires informative DIP alleles |
DIP-STR markers demonstrate approximately 100 times greater sensitivity in detecting minor DNA components in mixtures compared to standard STR profiling methods [48]. This exceptional sensitivity enables the detection of a donor contributing only 0.1% to mixed forensic evidence [9]. In practical applications, DIP-STRs have successfully detected minor DNA in challenging "touch" DNA samples where conventional STRs failed, demonstrating their utility for casework evidence such as handled objects [24].
Analysis of Degraded and Challenging Samples
The performance of genetic markers is heavily influenced by DNA quality, particularly in forensic contexts where samples may be degraded or inhibited.
Table 2: Performance with Challenging Forensic Samples
| Characteristic | Sequencing-Based STR | DIP Panels |
|---|---|---|
| Amplicon Size | Varies by multiplex system | Short (146-271 bp for forensic sets) [48] |
| Degradation Resistance | Moderate (depends on target sizes) | High (due to short amplicons) [48] [57] |
| PCR Artifacts | Stutter peaks still present [1] | Minimal stutter (non-dinucleotide repeats) [48] |
| Inhibition Tolerance | Platform-dependent | Validated for forensic samples [6] |
The development of DIP-STR markers with short amplicon sizes (146-271 bp) specifically addresses the challenge of analyzing degraded DNA evidence [48]. Furthermore, DIP-STRs that exclude dinucleotide repeats demonstrate minimal stutter peaks, improving typing accuracy and mixture interpretation [6] [48]. Validation studies confirm that DIP panels maintain reliable performance with inhibited forensic samples and low-quantity DNA inputs as minimal as 125 pg [57].
Forensic Informativeness and Statistical Power
Both technologies provide quantitative data for statistical evaluation of forensic evidence, though through different genetic mechanisms.
Sequencing-based STR typing benefits from established population databases and high heterozygosity of STR markers. The sequence-level polymorphism provides increased discriminatory power compared to length-based STR typing alone. However, statistical interpretation of mixtures remains complex, particularly for unbalanced samples.
DIP-STR markers are highly polymorphic, autosomal compounds that generate haplotypes with high discrimination power [4] [48]. A set of 10 DIP-STRs can produce random match probabilities in the range of 10^(-10) to 10^(-13) [48], providing strong statistical weight for evidence. The compound nature of DIP-STRs, combining biallelic DIPs with multiallelic STRs, creates a highly polymorphic system suitable for identity testing [4]. For personal identification, 60-plex DIP panels demonstrate combined probability of discrimination values approaching 1 and cumulative probability of paternity exclusion of 0.9937 [6].
Experimental Protocols and Methodologies
DIP-STR Typing Protocol
The experimental workflow for DIP-STR analysis follows a standardized molecular biology approach compatible with most forensic laboratory infrastructures.
Table 3: Key Research Reagent Solutions for DIP-STR Analysis
| Reagent/Category | Specific Examples/Requirements | Function in Experiment |
|---|---|---|
| DNA Extraction | QIAamp DNA Investigator Kit [57] | Isolation of high-quality genomic DNA from forensic samples |
| Quantification | Qubit dsDNA HS Assay Kit [57] | Accurate DNA quantification prior to amplification |
| PCR Amplification | Allele-specific primers for DIP, STR primer [48] | Target amplification of DIP-STR loci |
| Thermal Cycler | GeneAmp PCR system 9700 [57] | Precise temperature cycling for PCR |
| Separation System | 3500xL Genetic Analyzer [57] | Capillary electrophoresis for fragment separation |
| Analysis Software | GeneMapper ID-X [57] | Genotype determination and analysis |
Sample Preparation and DNA Extraction: Begin with DNA extraction from forensic samples (blood, saliva, touch DNA) using standardized forensic kits such as the QIAamp DNA Investigator Kit. Quantify DNA using fluorescent-based methods (e.g., Qubit dsDNA HS Assay) to ensure accurate input amounts [57].
PCR Amplification:
- Reaction Setup: Typical 10-25 μL reactions containing 1× reaction mix, 0.5-1.0 μM of each primer, 0.03-0.1 ng of minor DNA mixed with excess major DNA (up to 1000-fold), and PCR-grade water [48].
- Primer Design: Two allele-specific forward primers (for insertion "L" and deletion "S" alleles) and one common reverse primer located downstream of the STR [4] [48].
- Thermal Cycling Conditions: Initial denaturation at 94°C for 2-10 minutes; 25-30 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 1-2 minutes, extension at 72°C for 1 minute; final extension at 72°C for 10-30 minutes [48] [57].
Detection and Analysis:
- Separate PCR products using capillary electrophoresis systems (e.g., 3500xL Genetic Analyzer).
- Analyze results with fragment analysis software (e.g., GeneMapper ID-X) with peak detection thresholds typically set at 100 RFU [57].
- Determine informative markers where minor contributor possesses unique DIP alleles not present in major profile [24].
The experimental workflow for DIP-STR analysis emphasizes allele-specific amplification to selectively target the minor DNA component in a mixture.
Informative Genotype Determination
The successful application of DIP-STRs depends on identifying "informative" genotypes where the minor DNA contributor possesses DIP alleles not shared with the major contributor. This occurs in two primary scenarios:
- The major contributor is DIP homozygous (SS or LL) and the minor contributor is DIP heterozygous (SL) [1] [18].
- The major and minor contributors are opposite DIP homozygous (SS/LL or LL/SS) [1] [18].
When the minor DNA contributor has no unique S or L allele, DIP-STR typing resembles standard STR analysis and loses its specificity advantage for mixture resolution [1]. Therefore, population data and careful marker selection are crucial for effective implementation.
Application Scope and Limitations
Forensic and Medical Applications
Sequencing-Based STR Applications:
- High-throughput reference database generation
- Familial searching and kinship analysis
- Ancestry inference and biogeographical origin prediction
- Mutation studies and genetic genealogy
DIP-STR Panel Applications:
- Forensic Casework: Sexual assault evidence with male minor DNA mixed with female major DNA [2] [24]; "Touch" DNA from handled objects [24] [48]; Cases with same-sex contributors where Y-STRs are not applicable [24].
- Medical Genetics: Noninvasive prenatal diagnosis through detection of fetal DNA in maternal plasma [4] [18]; DNA microchimerism analysis following pregnancy or organ transplantation [4].
- Ancestry Inference: Biogeographic origin prediction using specifically selected DIP markers [6] [57].
Technology Limitations and Considerations
Sequencing-Based STR Limitations:
- Higher infrastructure costs and bioinformatics requirements [4]
- Persistence of PCR amplification bias in unbalanced mixtures [4]
- Complex mixture interpretation despite deeper sequencing
- Longer turnaround times for data analysis
DIP-STR Panel Limitations:
- Requires prior knowledge of major contributor's genotype for optimal marker selection [2]
- Limited multiplex capabilities currently (though expanding) [9]
- Dependence on population-specific databases for informative allele frequencies [1]
- Lower discrimination power per marker compared to standard STRs [48]
The choice between sequencing-based STR typing and DIP panel typing depends fundamentally on the specific forensic application and sample characteristics. For high-throughput analysis of single-source or moderately mixed samples, and for applications requiring ancestral information, sequencing-based STR methods provide comprehensive genetic information. However, for resolving extremely unbalanced DNA mixtures—particularly in "touch" DNA evidence and sexual assault cases with same-sex contributors—DIP panels offer unparalleled sensitivity and specificity.
Future developments in both technologies will continue to enhance forensic capabilities. For sequencing-based STRs, improved bioinformatic tools for mixture deconvolution and reduced costs will expand applications. For DIP panels, research focuses on developing expanded multiplex assays [9], population-specific marker sets, and integration with massively parallel sequencing platforms [9]. The combination of DIP markers with sequencing technologies may eventually provide the dual benefits of extreme sensitivity and high informativeness.
For forensic laboratories prioritizing the analysis of challenging mixed samples, implementing DIP-STR typing provides a powerful complementary tool to standard STR profiling, potentially enabling the resolution of cases that would otherwise yield inconclusive results. The straightforward workflow, compatibility with existing capillary electrophoresis infrastructure, and demonstrated sensitivity make DIP panels a valuable addition to the forensic genotyping toolkit.
Forensic genetics faces significant challenges when analyzing biological evidence from sexual assault cases and 'touch' DNA samples, which often contain DNA from multiple contributors in highly unbalanced ratios. In sexual assault evidence, mixtures typically contain a vast excess of the victim's epithelial cells compared to the perpetrator's sperm cells [58]. Similarly, 'touch' DNA evidence often comprises minimal biological material from a person of interest mixed with substantially more DNA from the primary handler of an item [9]. These unbalanced mixtures pose considerable difficulties for conventional Short Tandem Repeat (STR) profiling, which struggles to detect minor DNA contributors representing less than 10% of the total DNA [4]. This case study provides a comparative analysis of the performance between innovative Deletion/Insertion Polymorphism-STR (DIP-STR) panels and traditional STR markers, presenting experimental data on their efficacy, sensitivity, and application success rates in forensic contexts.
Marker Technology Comparison: STR vs. DIP-STR
Fundamental Principles and Mechanisms
Traditional STR Markers consist of short, repetitive DNA sequences (typically 2-6 base pairs) that are highly polymorphic due to variation in repeat number. Standard forensic analysis involves PCR amplification followed by capillary electrophoresis to separate and detect length variants [14]. Conventional STR profiling encounters limitations with unbalanced mixtures due to PCR amplification bias, where the major DNA component amplifies preferentially, masking the minor contributor's alleles. This method generally requires the minor DNA component to constitute at least 10-20% of the total DNA for reliable detection [4].
DIP-STR Markers are compound genetic markers that pair a deletion/insertion polymorphism (DIP) with a closely linked STR. The innovative design utilizes allele-specific PCR primers that overlap the DIP variant, enabling targeted amplification of the minor contributor's DNA by exploiting genotype differences between mixture components [4]. This approach generates haplotypes (combinations of DIP and STR alleles) that provide high polymorphism for individual identification. The compound nature of these markers facilitates analysis of extremely unbalanced mixtures where traditional STRs fail [9].
Comparative Performance Metrics
Table 1: Direct Performance Comparison Between STR and DIP-STR Markers
| Performance Characteristic | Traditional STR Markers | DIP-STR Markers |
|---|---|---|
| Minimum Detectable Minor Contributor Ratio | 1:10 to 1:20 [4] | Up to 1:1000 [4] [9] |
| Typical Amplicon Size Range | 100-500 bp [14] | 70-325 bp (average ~230 bp) [9] |
| Required DNA Input | 125-1000 pg [23] | As low as 60 pg [9] |
| Mixed Sample Analysis | Limited in highly unbalanced mixtures [4] | Excellent for unbalanced two-person mixtures [9] |
| Degraded DNA Performance | Variable, dependent on amplicon size [14] | Enhanced through shorter amplicons [9] |
| Mutation Rate | ~0.1% per locus per generation [14] | DIP component: ~10⁻⁸ [7] |
Table 2: Success Rates in Forensic Applications
| Application Scenario | STR Success Rate | DIP-STR Success Rate | Notes |
|---|---|---|---|
| Sexual Assault Evidence (Male DNA in Female Background) | ~72% (with differential extraction) [58] | >90% (at 1:1000 ratios) [4] | Y-STRs are limited to male-minor mixtures [4] |
| Touch DNA Mixtures | Limited by low template and mixture ratio [9] | High (minor contributor 0.1%) [9] | DIP-STRs work irrespective of contributor sexes [9] |
| DNA Mixture Deconvolution | Partial profiles common in unbalanced mixtures [14] | Full minor contributor profiles achievable [9] | DIP-STRs enable unambiguous genotyping [4] |
| Analysis of Degraded Samples | Dependent on degradation level and amplicon size [14] | Improved recovery with <200 bp amplicons [7] | 80% of DIP-STRs are <200 bp [23] |
Experimental Protocols and Methodologies
DIP-STR Analysis Workflow
The following diagram illustrates the experimental workflow for DIP-STR analysis of unbalanced DNA mixtures:
Detailed Experimental Protocol for DIP-STR Analysis
Sample Preparation and DNA Extraction:
- Extract DNA using standard forensic protocols (organic, Chelex, or automated systems)
- Quantify DNA using fluorescence-based methods (e.g., Qubit, PicoGreen)
- For sexual assault evidence, differential extraction is recommended to separate sperm and epithelial cell fractions [58]
- Input DNA amounts can range from 0.06 ng to 1 ng, with optimal performance at 0.5-1 ng [9]
Allele-Specific PCR Amplification:
- Design primers with 3' ends overlapping the DIP variant to ensure allele specificity
- Include one primer complementary to the deletion (S-DIP) and another complementary to the insertion (L-DIP)
- The non-allele specific primer targets the flanking STR region
- Reaction conditions: 10-25 μL reaction volume, 25-30 amplification cycles [9]
- Thermal cycling parameters: initial denaturation at 94°C for 2 minutes; followed by cycles of 94°C for 30 seconds, 58-62°C for 30 seconds, 72°C for 45 seconds; final extension at 72°C for 10 minutes [4]
Capillary Electrophoresis and Data Analysis:
- Separate PCR products using capillary electrophoresis systems (e.g., ABI 3500 series)
- Include appropriate size standards for accurate fragment sizing
- Analyze data with genotyping software, applying analytical thresholds of 50-100 RFU
- Interpret results based on haplotype profiles, comparing major and minor contributor patterns [9]
Validation and Quality Control:
- Include positive controls (known genotype reference samples) and negative controls (no template) in each run
- Assess sensitivity using serial dilutions of control DNA
- Evaluate specificity with mixed samples at varying ratios
- Determine stochastic effects and limits of detection [9]
Research Reagent Solutions
Table 3: Essential Research Reagents for DIP-STR Analysis
| Reagent/Category | Specific Examples | Function and Application Notes |
|---|---|---|
| DNA Extraction Systems | Biomek NXP Robotic Platform [58], Qiagen EZ1 DNA Investigator | Automated differential extraction for sexual assault evidence; optimized for low-input samples |
| PCR Amplification Kits | AmpliTaq Gold DNA Polymerase, QIAGEN Multiplex PCR Kit | Hot-start enzymes for specificity; optimized buffer systems for multiplex reactions |
| Capillary Electrophoresis Kits | ABI PRISM 310 Genetic Analyzer, Applied Biosystems 3500 Series | Fragment separation and detection; multiple fluorescent dye capabilities |
| Commercial MPS Systems | MiSeq FGx Forensic Genomics System [23], Ion Torrent PGM | Massively parallel sequencing for high-throughput DIP-STR analysis |
| DIP-STR Primer Panels | Custom-designed allele-specific primers [9] | Target specific DIP-STR loci; designed for minimal primer-dimer formation |
| Quantification Assays | Qubit dsDNA HS Assay, Quantifiler Trio DNA Quantification Kit | Accurate DNA quantification; assessment of DNA quality and inhibitor presence |
Case Study Data: Sexual Assault Evidence Analysis
Performance in Actual Casework
A comprehensive validation study demonstrated the superior performance of DIP-STR markers in resolving challenging forensic mixtures. The research analyzed 30 DIP-STR markers across 103 Swiss individuals, reporting high sensitivity and specificity even with minimal DNA input [9]. Key findings included:
- Sensitivity: Full profiles obtained with as little as 0.06 ng of DNA input
- Specificity: Successful detection of minor DNA contributors representing only 0.1% of the mixture
- Reproducibility: Consistent results across replicate analyses and different operators
- Population Genetics: Average heterozygosity of 0.7 across the marker set, indicating high discriminatory power [9]
In sexual assault case simulations, DIP-STR markers successfully generated complete profiles of minor male contributors in female background at ratios up to 1:1000, whereas standard STR analysis failed to detect any minor contributor alleles at ratios beyond 1:20 [4].
Impact on Justice Outcomes
The effectiveness of DNA evidence significantly impacts sexual assault prosecutions. Research examining over 100 sexual assault cases accepted for prosecution found that:
- Cases with DNA evidence matching suspects were more likely to be prosecuted (75% vs. <33%)
- Conviction odds were more than nine times greater with DNA evidence [59]
- Backlog reduction through automated processing increases efficiency and potentially prevents additional crimes by recidivist offenders [60]
Advanced Applications and Future Directions
Ancestry Inference Capabilities
Beyond mixture deconvolution, DIP-STR markers show promise for biogeographical ancestry inference. A study evaluating 23 DIP-STR markers in HGDP-CEPH reference samples demonstrated their ability to distinguish seven major population groups with accuracy comparable to existing ancestry-informative marker panels [61]. The compound nature of DIP-STRs combines the evolutionary stability of DIPs (suitable for studying longer-term population history) with the higher mutation rate of STRs (providing resolution for recent demographic events) [61].
Recent research has developed a 60-marker DIP panel specifically designed for forensic ancestry inference and personal identification in East Asian populations. This panel demonstrated:
- Combined probability of discrimination: 0.999999999999
- Cumulative probability of paternity exclusion: 0.9937
- Robust performance with degraded DNA samples [7]
Integration with Massively Parallel Sequencing
The future of DIP-STR analysis lies in integration with massively parallel sequencing (MPS) technologies. MPS enables simultaneous analysis of thousands of genetic markers, providing high-resolution data from challenging samples [23]. Commercial MPS solutions specifically designed for forensic applications include:
- Illumina/Verogen MiSeq FGx System: Sequences up to 231 markers (STRs and SNPs) in a single run, specifically validated for forensic casework [23]
- Thermo Fisher Scientific's Precision ID Panels: Target STRs, SNPs, and mitochondrial DNA with minimal DNA input requirements (as low as 2 pg for mtDNA analysis) [23]
This technological synergy between DIP-STR markers and MPS platforms represents the cutting edge of forensic genetics, potentially overcoming current limitations in mixture deconvolution and ancestry inference.
This comparative analysis demonstrates that DIP-STR markers significantly outperform traditional STR profiling in handling the challenging DNA evidence typical of sexual assault cases and touch DNA samples. The experimental data confirm that DIP-STR technology can successfully resolve extremely unbalanced mixtures (up to 1:1000 ratios) that routinely defeat conventional STR analysis. The method's sensitivity with low DNA inputs (as little as 0.06 ng) and degraded samples, combined with its ability to generate complete profiles of minor contributors, positions DIP-STR analysis as a transformative approach in forensic genetics. As the field advances, integration with massively parallel sequencing platforms and expansion of validated marker panels will further enhance the success rates of DNA evidence analysis in sexual assault investigations and other forensic contexts where mixture deconvolution remains challenging.
Conclusion
The comparative analysis reveals that DIP panels and STR markers are not mutually exclusive but rather complementary tools in the modern forensic geneticist's arsenal. STRs remain the robust, standardized foundation for routine DNA profiling, while DIPs, particularly in compound DIP-STR forms, offer unparalleled advantages for specific, high-challenge scenarios such as extremely unbalanced mixtures, degraded samples, and ancestry inference. The superior sensitivity of DIP-STRs, capable of detecting a minor DNA contributor at ratios up to 1:1000, fills a critical gap that traditional STRs cannot address. Future directions point toward the integration of these markers into massively parallel sequencing (MPS) workflows, the development of expanded, population-specific multiplex panels, and their growing application in biomedical fields such as non-invasive prenatal diagnosis and transplantation monitoring. Embracing this multi-marker approach will be pivotal for advancing the resolution, reliability, and scope of forensic and clinical genetic analyses.
References
- 1. Application of Y-STR, DIP-STR and SNP-STR Markers in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9529747/]
- 2. An investigation of the potential of DIP-STR markers for ... [https://www.sciencedirect.com/science/article/abs/pii/S1872497314000775]
- 3. Utility of regional STR marker variations in Tunisian and ... [https://www.frontiersin.org/journals/bioinformatics/articles/10.3389/fbinf.2025.1550730/full]
- 4. DIP–STR: Highly Sensitive Markers for the Analysis of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3675636/]
- 5. Forensic applications of rapidly mutating Y-STR markers [https://pubmed.ncbi.nlm.nih.gov/41148463/?utm_source=FeedFetcher&utm_medium=rss&utm_campaign=None&utm_content=0TFakvcJ1oHKSxwfjcNlEsnaJGLJS4ZAI_ANm8UruRd&fc=None&ff=20251029043510&v=2.18.0.post22+67771e2]
- 6. Establishment and validation of a DIP panel for forensic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11927128/]
- 7. Establishment and validation of a DIP panel for forensic ancestry... [https://humgenomics.biomedcentral.com/articles/10.1186/s40246-025-00727-8]
- 8. Forensic DNA Profiling: Autosomal Short Tandem Repeat ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7444828/]
- 9. Identification and characterization of novel DIP-STRs from ... [https://www.sciencedirect.com/science/article/abs/pii/S1872497323000248]
- 10. Establishment and validation of a DIP panel for forensic ... [https://pubmed.ncbi.nlm.nih.gov/40119476/]
- 11. Accurate profiling of forensic autosomal STRs using the ... [https://www.sciencedirect.com/science/article/abs/pii/S1872497321001654]
- 12. Review Forensic applications of compound genetic markers [https://www.sciencedirect.com/science/article/abs/pii/S1355030625000851]
- 13. Genetic Diversity of 10 DIP-STR Markers in US Groups [https://bioengineer.org/genetic-diversity-of-10-dip-str-markers-in-us-groups/]
- 14. Enhancing Forensic DNA Profiling Efficiency [https://promega.foleon.com/theishireport/the-ishi-report-august-2025/enhancing-forensic-dna-profiling-efficiency-through-alternative-str-based-multiplex-systems]
- 15. Nanopore sequencing of MiniHap biomarkers for forensic ... [https://pubmed.ncbi.nlm.nih.gov/40106854/]
- 16. Forensic efficiencies of individual identification, kinship ... [https://www.frontiersin.org/journals/genetics/articles/10.3389/fgene.2022.1057231/full]
- 17. Autosomal DIPs for population genetic structure and ... [https://www.nature.com/articles/s41598-018-29010-8]
- 18. Genotyping of STR and DIP–STR Markers in Plasma Cell ... [https://www.cambridge.org/core/journals/twin-research-and-human-genetics/article/genotyping-of-str-and-dipstr-markers-in-plasma-cellfree-dna-for-simple-and-rapid-noninvasive-prenatal-diagnosis-of-zygosity-of-twin-pregnancies/77BCDED5587711975251EEF1C8695022]
- 19. Genetic data and population structure of 10 DIP-STR ... [https://pubmed.ncbi.nlm.nih.gov/41249519/]
- 20. Short tandem repeat markers in diagnostics: what's in a ... [https://www.nature.com/articles/2404273]
- 21. Short tandem repeat (STR) DNA markers are hypervariable ... [https://pubmed.ncbi.nlm.nih.gov/12505473/]
- 22. Human de novo mutation rates from a four-generation ... [https://www.nature.com/articles/s41586-025-08922-2]
- 23. Applications of massively parallel sequencing in forensic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9514793/]
- 24. Sensitive DIP-STR markers for the analysis of unbalanced ... [https://www.sciencedirect.com/science/article/abs/pii/S1872497317300339]
- 25. Protocols for STR Analysis Manuals - OCME [https://www.nyc.gov/site/ocme/services/fbio-2025-protocols-for-str-analysis-manuals.page]
- 26. Performance of two 17 locus forensic identification STR kits ... [https://www.sciencedirect.com/science/article/abs/pii/S1872497311002365]
- 27. An investigation of a set of DIP-STR markers to detect ... [https://www.sciencedirect.com/science/article/abs/pii/S1872497317301837]
- 28. Investigation into the genotyping performance of a unique ... [https://www.sciencedirect.com/science/article/abs/pii/S187249732500016X]
- 29. Pattern Analysis of Short Tandem Repeats Allele ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6252038/]
- 30. Systematic evaluations of forensic effectiveness and genetic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12083099/]
- 31. A forensic population database of autosomal STR and X ... [https://www.sciencedirect.com/science/article/pii/S240584402309031X]
- 32. Short Tandem Repeats (STRs) as Biomarkers for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7565503/]
- 33. Noninvasive Prenatal Testing: The Future Is Now - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3893900/]
- 34. Fetomaternal microchimerism and genetic diagnosis [https://www.sciencedirect.com/science/article/pii/S1383574221000363]
- 35. Comparison of Chromosomal Microarray Analysis and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10775152/]
- 36. Performance analysis of non-invasive prenatal testing for ... [https://www.sciencedirect.com/science/article/pii/S2405844024094684]
- 37. Chimerism detection by short tandem repeat analysis when ... [https://www.sciencedirect.com/science/article/abs/pii/S0009898111006565]
- 38. Abundance and amplification bias in amplicon sequencing [https://www.drive5.com/usearch/manual/amplification_bias.html]
- 39. Bias in Template-to-Product Ratios in Multitemplate PCR [https://pmc.ncbi.nlm.nih.gov/articles/PMC106531/]
- 40. A mixture detection method based on separate ... [https://pubmed.ncbi.nlm.nih.gov/28119211/]
- 41. Analyzing and minimizing PCR amplification bias in Illumina ... [https://genomebiology.biomedcentral.com/articles/10.1186/gb-2011-12-2-r18]
- 42. Degraded DNA Analysis Techniques: Purification, ... [https://www.sepscience.com/degraded-dna-analysis-techniques-purification-characterization-and-forensic-workflows-12020]
- 43. Comparison of different interpretation strategies for low ... [https://www.sciencedirect.com/science/article/abs/pii/S1872497312001391]
- 44. Setting the Standard, Ensuring Justice [https://artsandsciences.syracuse.edu/news-all/news-2025/setting-the-standard-ensuring-justice/]
- 45. Genomics will forever reshape forensic science and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12455754/]
- 46. Emerging Technologies in Forensic DNA Analysis [https://www.sciepublish.com/article/pii/279]
- 47. STR Data Analysis and Interpretation for Forensic Analysts [https://nij.ojp.gov/nij-hosted-online-training-courses/str-data-analysis-and-interpretation-forensic-analysts/data-troubleshooting/stutter]
- 48. A novel set of DIP-STR markers for improved analysis ... [https://www.sciencedirect.com/science/article/abs/pii/S1872497315300466]
- 49. Population-specific FST values for forensic STR markers [https://www.sciencedirect.com/science/article/abs/pii/S1872497316300370]
- 50. Population-specific FST values for forensic STR markers [https://pmc.ncbi.nlm.nih.gov/articles/PMC4899164/]
- 51. Algorithms for selecting informative marker panels ... [https://pubmed.ncbi.nlm.nih.gov/16305328/]
- 52. Validation of novel forensic DNA markers using multiplex ... [https://www.sciencedirect.com/science/article/pii/S1872497320300466]
- 53. Set of 15 SNP-SNP Markers for Detection of Unbalanced ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8866868/]
- 54. Systematic evaluations of forensic effectiveness and genetic ... [https://hereditasjournal.biomedcentral.com/articles/10.1186/s41065-025-00416-5]
- 55. Sensitivity, reproducibility, and accuracy in short tandem ... [https://pubmed.ncbi.nlm.nih.gov/8889558/]
- 56. Evaluation of a hybridization capture-based hereditary ... [https://www.sciencedirect.com/science/article/abs/pii/S0379073825002324]
- 57. Comprehensive evaluations of individual discrimination ... [https://hereditasjournal.biomedcentral.com/articles/10.1186/s41065-023-00271-2]
- 58. Automation of Sexual Assault DNA Processing Increases ... [https://nij.ojp.gov/topics/articles/automation-sexual-assault-dna-processing-increases-efficiency]
- 59. Study Examines Impact of DNA Evidence in Sexual Assault ... [https://socialwork.illinois.edu/2022/04/08/study-examines-impact-of-dna-evidence-in-sexual-assault-prosecutions/]
- 60. The value of forensic DNA leads in preventing crime and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8476691/]
- 61. Inferring biogeographic ancestry with compound markers of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6189140/]